touchCONGRESS Progress in the management of EGFR-mutant NSCLC in 2020: Where are we now?
Watch expert review and interpretation of the latest findings for the treatment of EGFR-mutant non-small cell lung cancer (NSCLC) in this webinar from the American Society of Clinical Oncology Virtual 2020 Conference (ASCO20 Virtual), 29–31 May 2020.
Part 1: Watch internationally renowned expert Dr Roy Herbst review the key data from ASCO20 Virtual
Part 2: Choose from leading experts who discuss what data finding mean for global and regional practice
Introduction and summary of key trials at ASCO20 Virtual
Will targeted therapies play a significant role in the adjuvant setting?
How is the treatment landscape evolving in metastatic EGFR-mutant NSCLC?
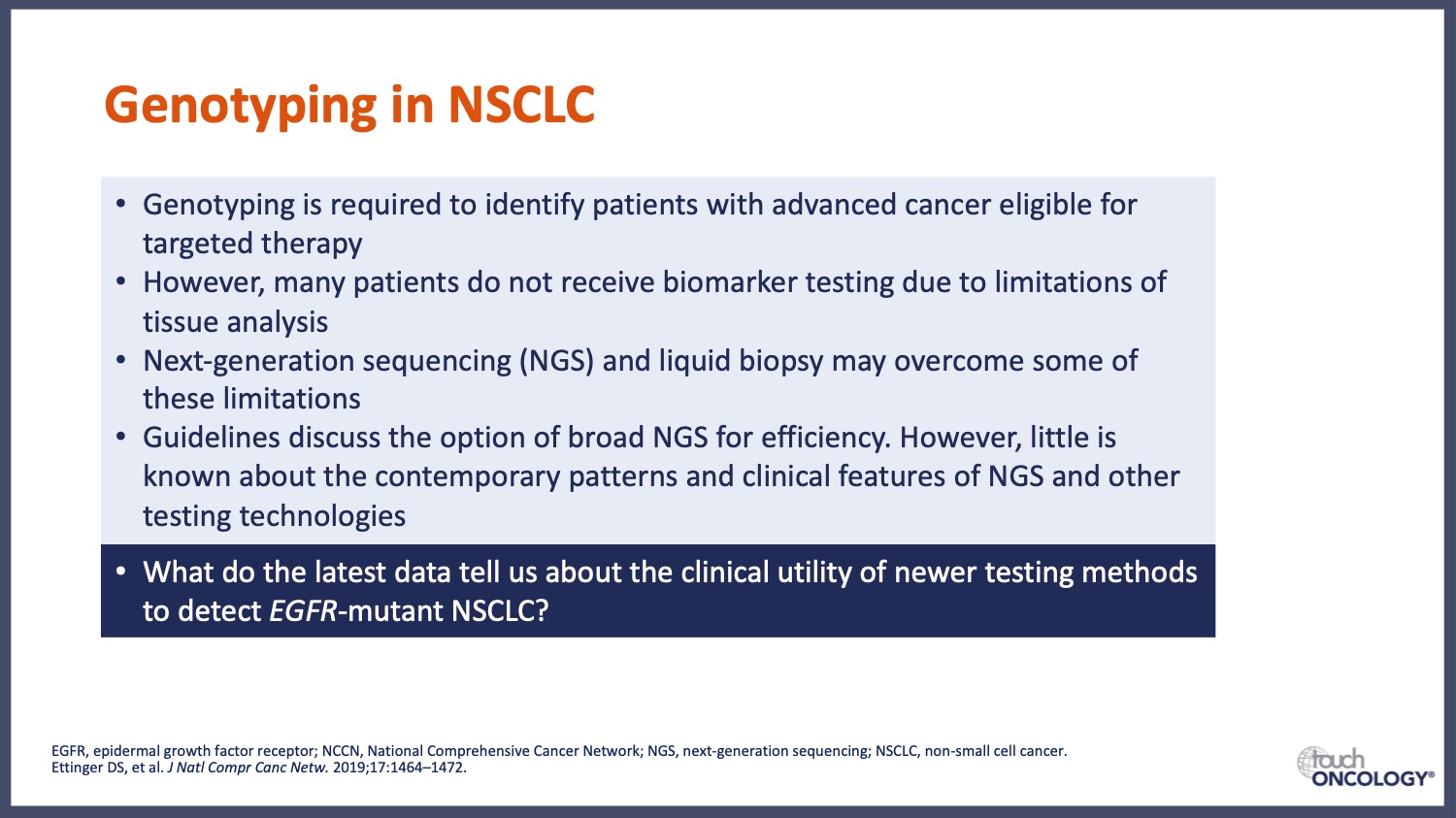
What advances in mutation testing will further support precision medicine in EGFR-mutant NSCLC?
Overview
Watch Dr Roy S Herbst discuss the optimal use of targeted therapy and genotyping for patients with EGFR-mutant NSCLC and consider the key research findings from ASCO20 Virtual, including:
- What does the latest data for tyrosine kinase inhibitors (TKIs) in patients with EGFR-mutant NSCLC tell us about their use in the adjuvant and metastatic settings? A focus on evidence from frontline studies
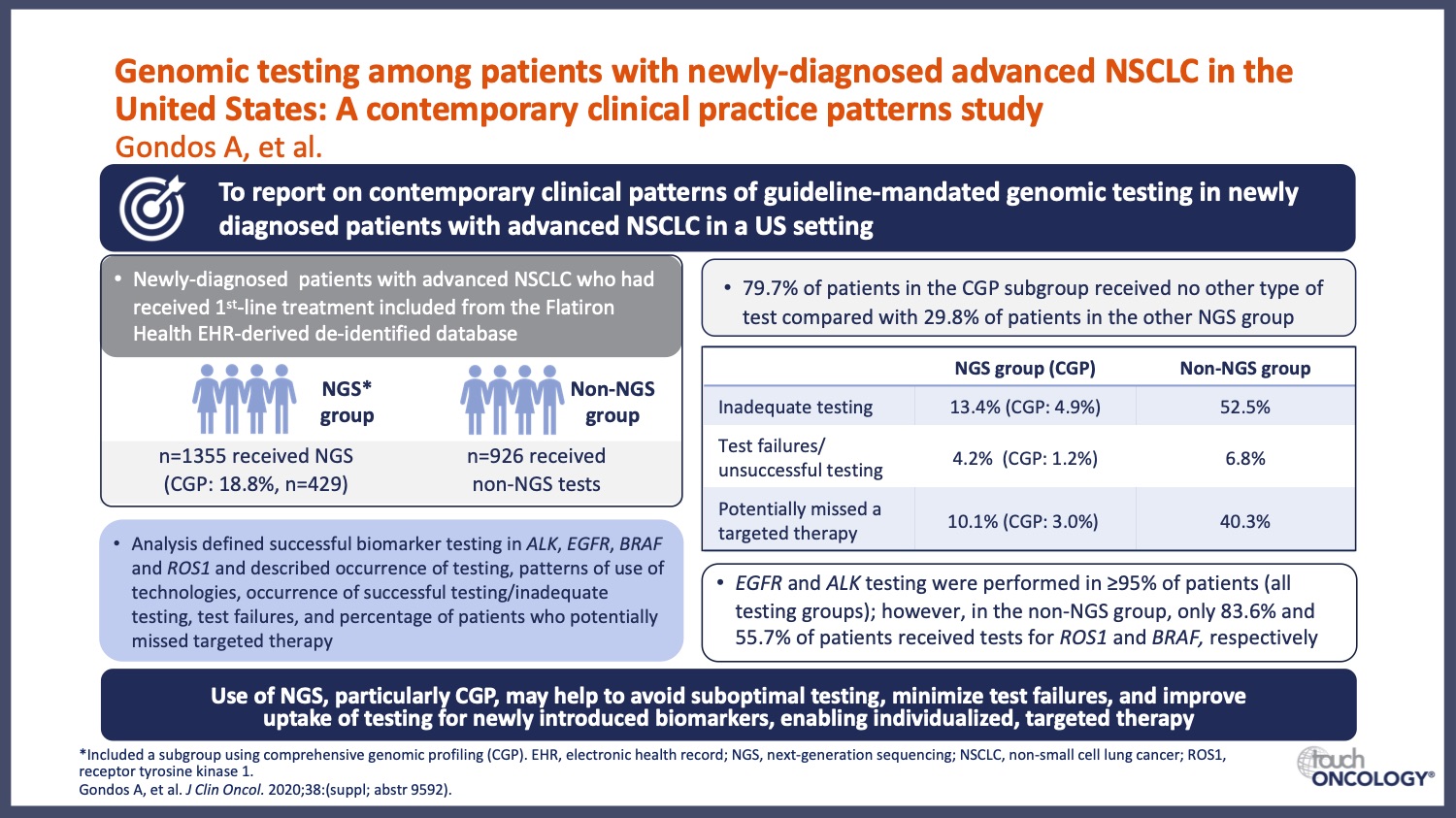
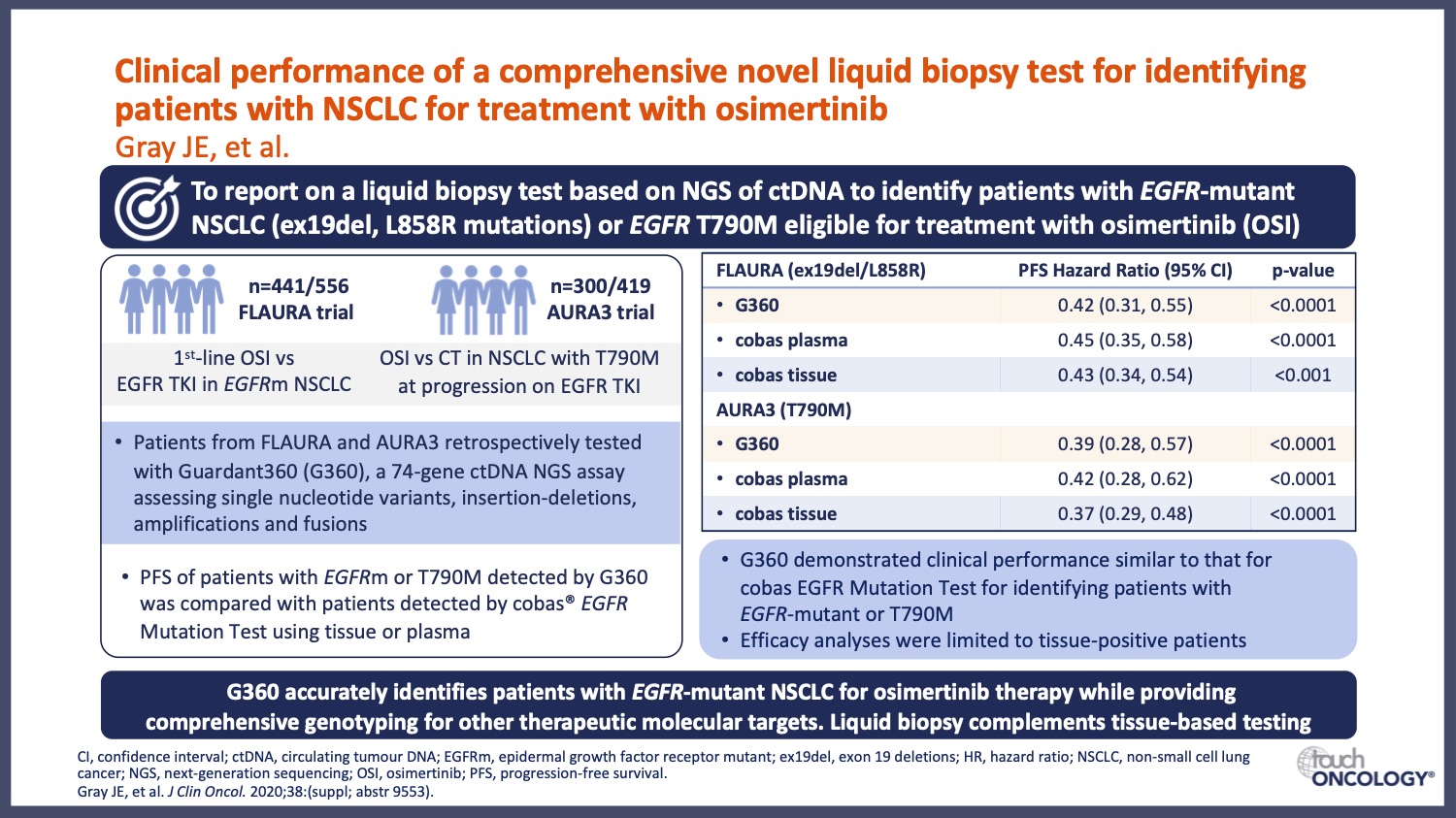
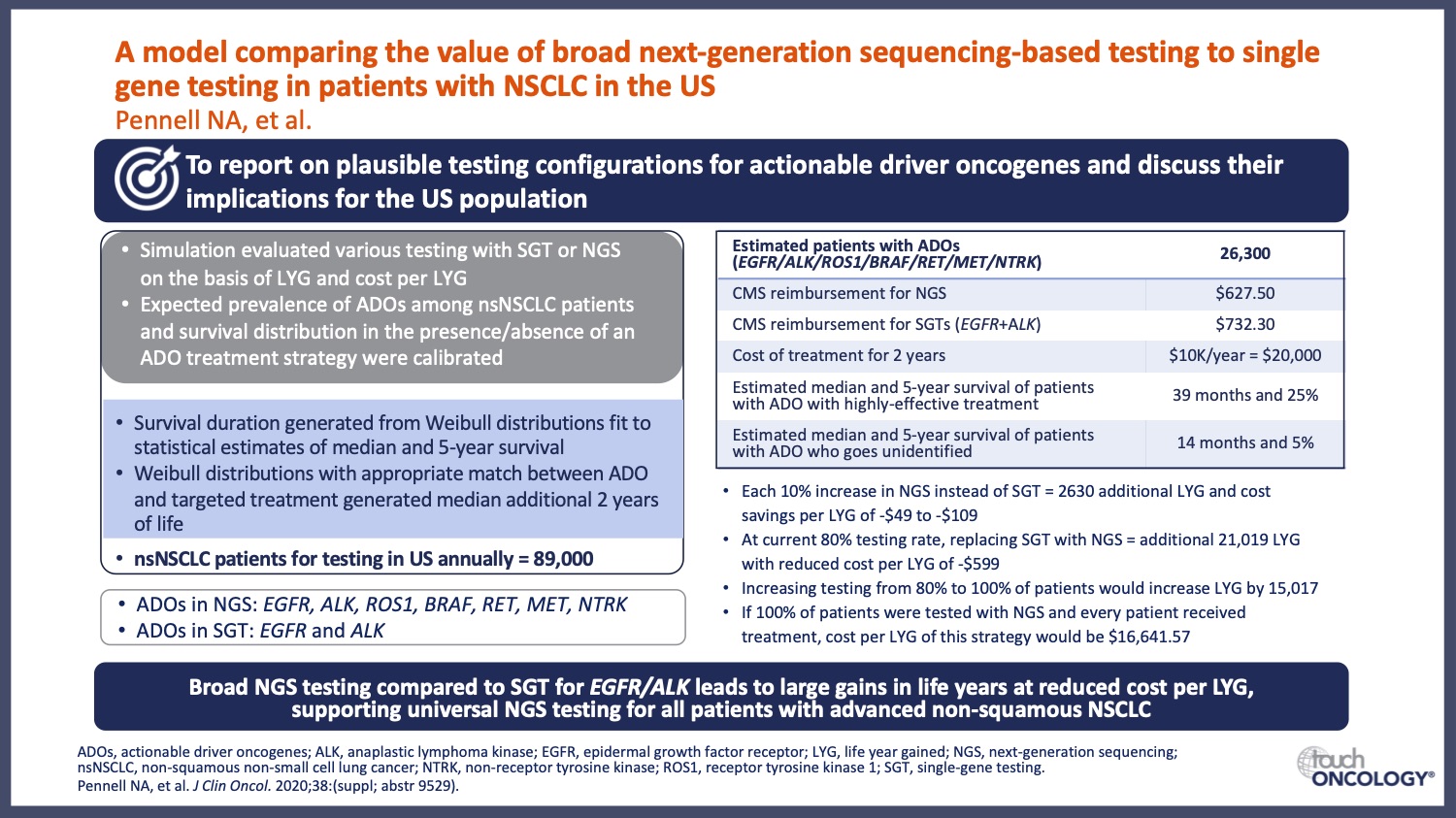
- How does new research advance the use of genomic testing in EGFR-mutant NSCLC? A focus on how clinical trial data are driving the collection of important genotyping information in NSCLC
- How is the treatment paradigm for EGFR-mutant NSCLC evolving? A focus on novel treatment approaches to guide current and future clinical decision making
Dr Roy Herbst is Professor of Medicine and Pharmacology, Chief of Medical Oncology, Director of the Thoracic Oncology Research Program, and Associate Director for Translational Research at Yale Cancer Center and Yale School of Medicine, New Haven, Connecticut in the USA.
Dr Herbst has worked as a pioneer of personalized medicine and immunotherapy to identify biomarkers and bring novel targeted treatments and immunotherapies to patients and has served as a principal investigator for numerous clinical trials testing these agents in advanced stage lung cancers. His work on “umbrella” trials has galvanized the field of targeted therapy and cancer drug approvals at the FDA.
Dr Herbst has been a champion of translational medicine, recently authoring a high-profile review of the 20-year progress in lung cancer. He has also authored or co-authored more than 300 publications, including peer-reviewed journal articles, abstracts and book chapters. For his lifetime achievement in scientific contributions to thoracic cancer research, Dr Herbst was awarded the 2016 Paul A. Bunn, Jr Scientific Award by the International Association for the Study of Lung Cancer (IASLC).
Dr Roy S Herbst discloses: Board Member (non‐executive/independent) for: Junshi Biosciences. Consulting for: AbbVie Pharmaceuticals, ARMO BioSciences, AstraZeneca, Biodesix, Bolt Biotherapeutics, Bristol‐Myers Squibb, Cybrexa Therapeutics, Eli Lilly and Company, EMD Serono, Genentech/Roche, Genmab, Halozyme Therapeutics, Heat Biologics, I-Mab Biopharma, Immunocore, Infinity Pharmaceuticals, Loxo Oncology, Merck & Co. Inc., Mirati Therapeutics, Inc., Nektar, Neon Therapeutics, NextCure, Novartis, Pfizer, Sanofi, Seattle Genetics, Shire Plc, Spectrum Pharmaceuticals, Symphogen, Takeda, Tesaro, Tocagen. Research Support from: AstraZeneca, Eli Lilly and Company, Genentech/Roche, Merck & Co. Inc.
Prof. Roy S Herbst, considers the impact of emerging data presented at ASCO20 Virtual and discusses how they may influence treatment decisions for patients with EGFR-mutant NSCLC.
Prof. David Planchard, Associate Professor of Medicine, Thoracic Tumour Board, at the Institut Gustave Roussy, Villejuif, France, considers the impact of emerging data presented at ASCO20 Virtual and discusses how they may influence treatment decisions for patients with EGFR-mutant NSCLC.
Dr Shun Lu, Professor at Shanghai Chest Hospital, Jiao Tong University and Chief of Shanghai Lung Cancer Center, Shanghai, China, considers the impact of emerging data presented at ASCO20 Virtual and discusses how they may influence treatment decisions for patients with EGFR-mutant NSCLC.
Please Select A Video:
Overview & Learning Objectives
Overview
Watch expert review and interpretation of the latest findings for the treatment of EGFR-mutant non-small cell lung cancer (NSCLC) in this webinar from the American Society of Clinical Oncology Virtual 2020 Conference (ASCO20 Virtual), 29–31 May 2020.
The information in this activity is intended for oncologists, pulmonologists, oncology nurse specialists and other healthcare professionals involved in the treatment of patients with lung cancer.
Learning Objectives
After watching this touchCONGRESS Webinar, you should be able to:
- Recall efficacy and safety data for EGFR-TKIs for patients with EGFR-mutant NSCLC, including those with early-stage disease
- Describe how molecular testing can help guide appropriate therapeutic approaches for patients with EGFR-mutant NSCLC
- Discuss how the treatment pathway for the use of targeted therapies in the management of EGFR-mutant NSCLC is evolving now and in the future

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights