touchCONGRESS HR+/HER2- Advanced breast cancer: what are the latest developments in CDK4/6 inhibition from the ESMO 2019?
Hear expert review and interpretation of the latest findings for treating HR+/HER2- advanced breast cancer with CDK4/6 inhibitors in this webinar from the European Society for Medical Oncology (ESMO) Congress in Barcelona, Spain, 27 September–1 October 2019.
Part 1: Watch internationally renowned expert Dr Olivier Trédan review key data from ESMO 2019
Part 2: Choose from leading experts who discuss what the data findings mean for global and regional practice
Introduction
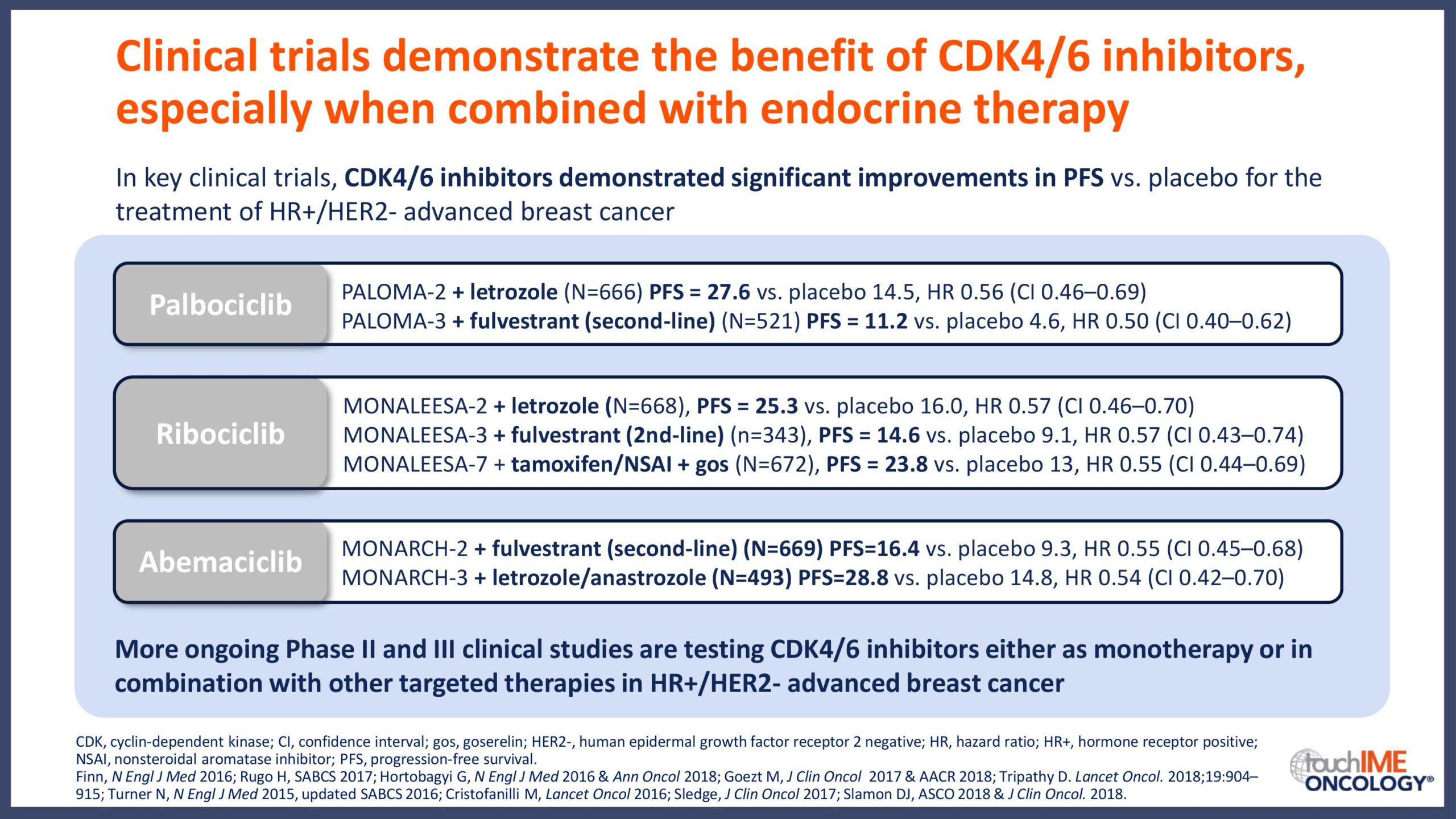
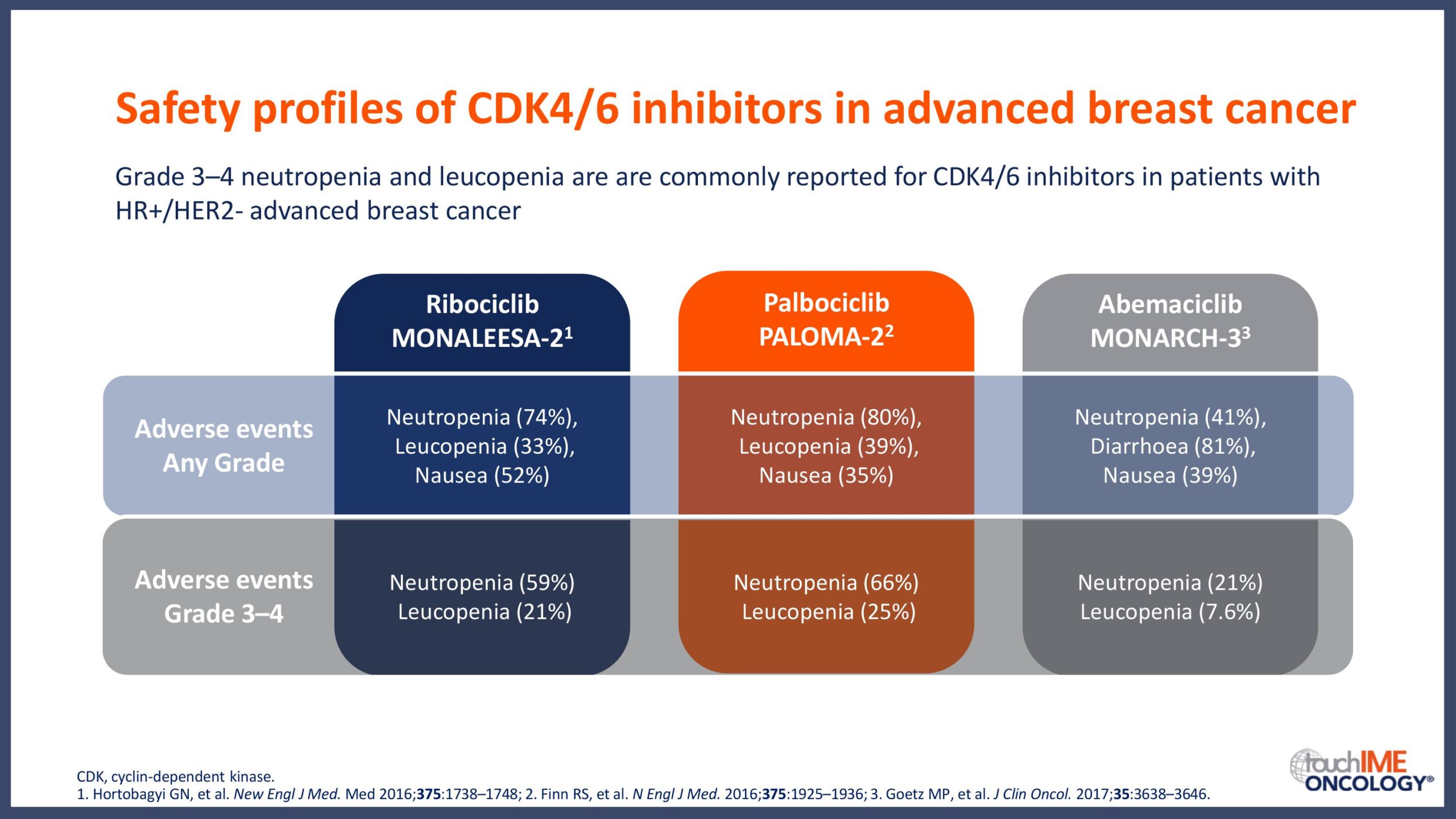
The clinical efficacy and safety of CDK4/6 inhibitors
ESMO Congress 2019 – What are the latest data for CDK4/6 inhibitors?
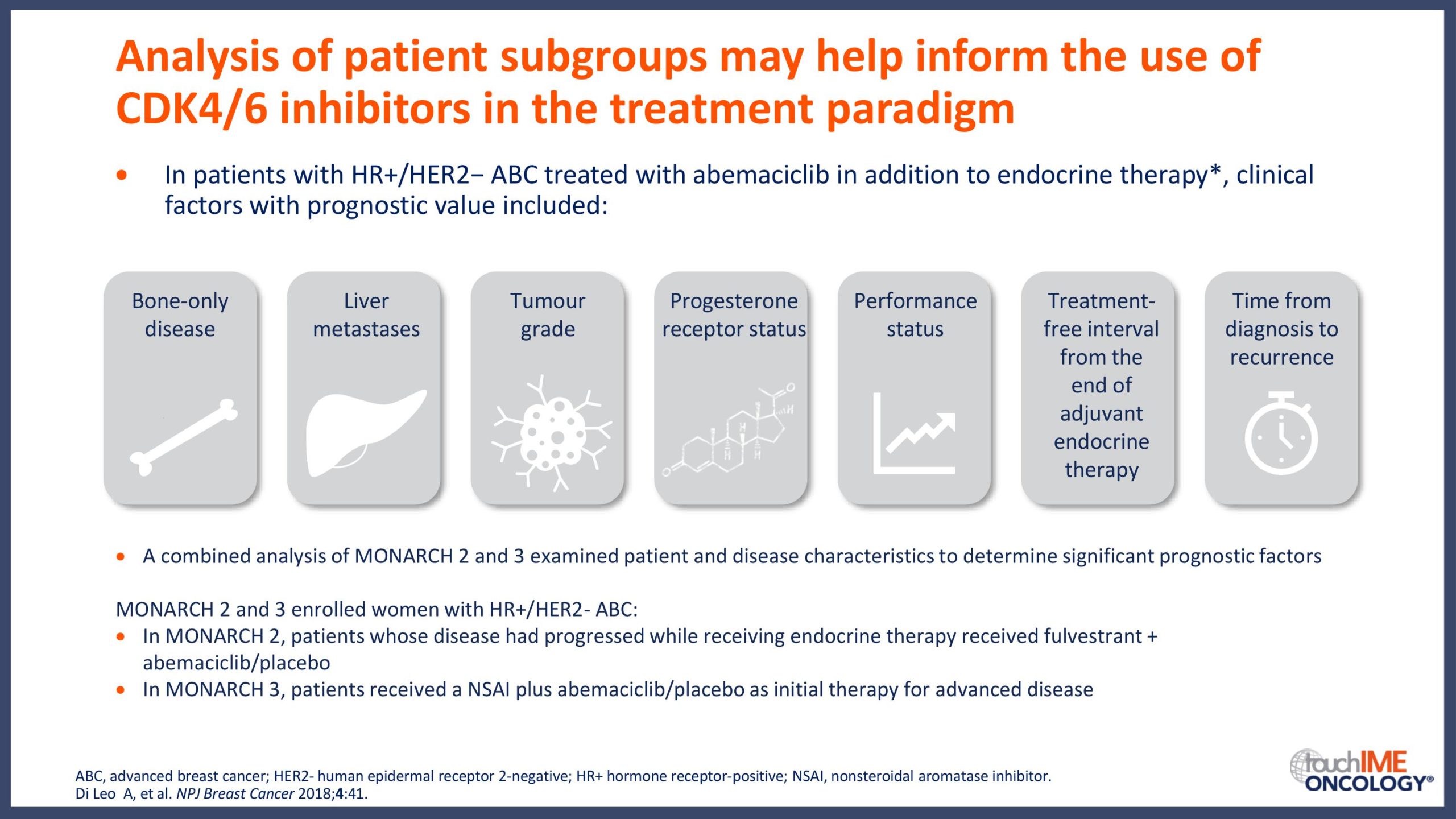
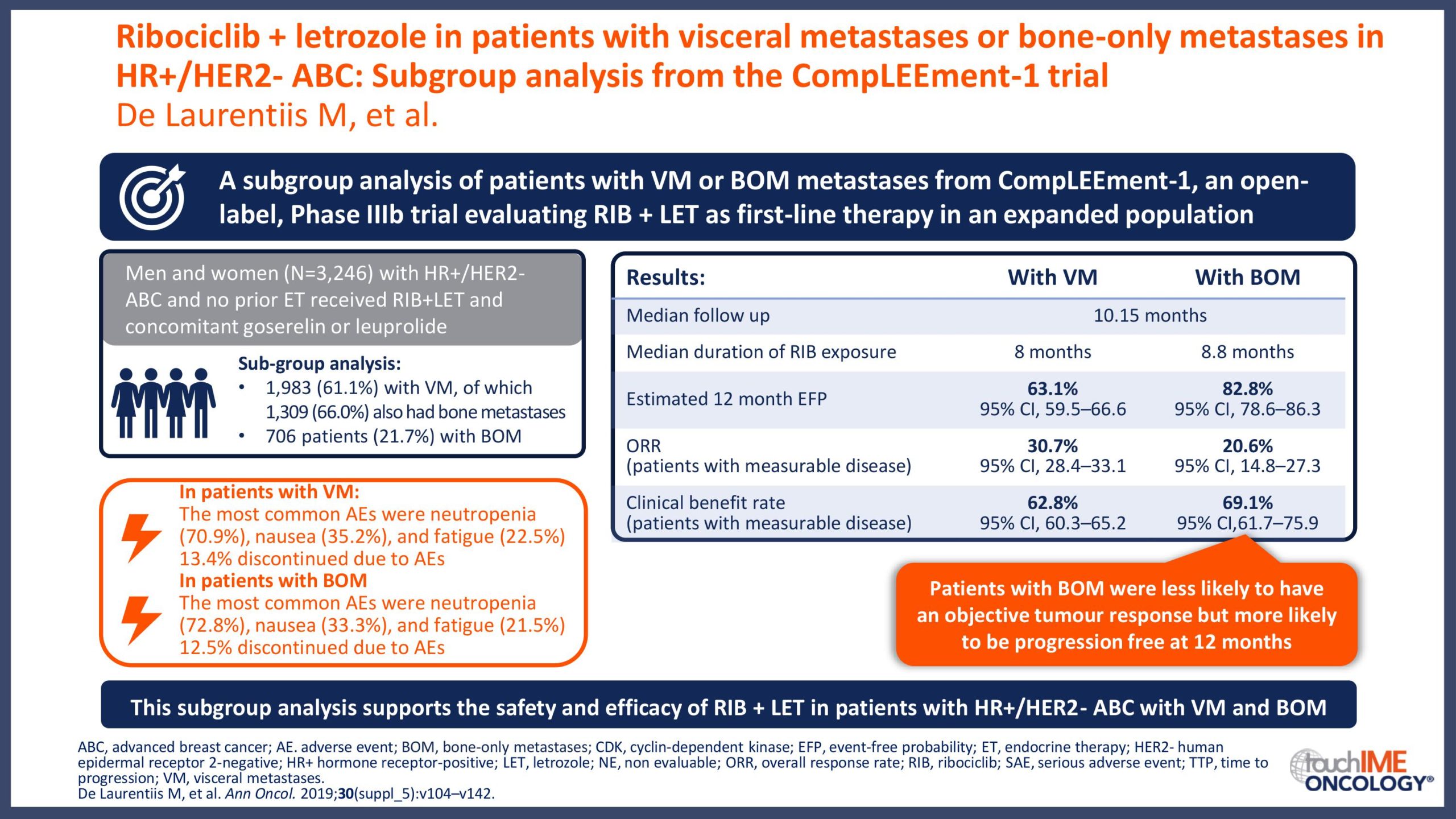
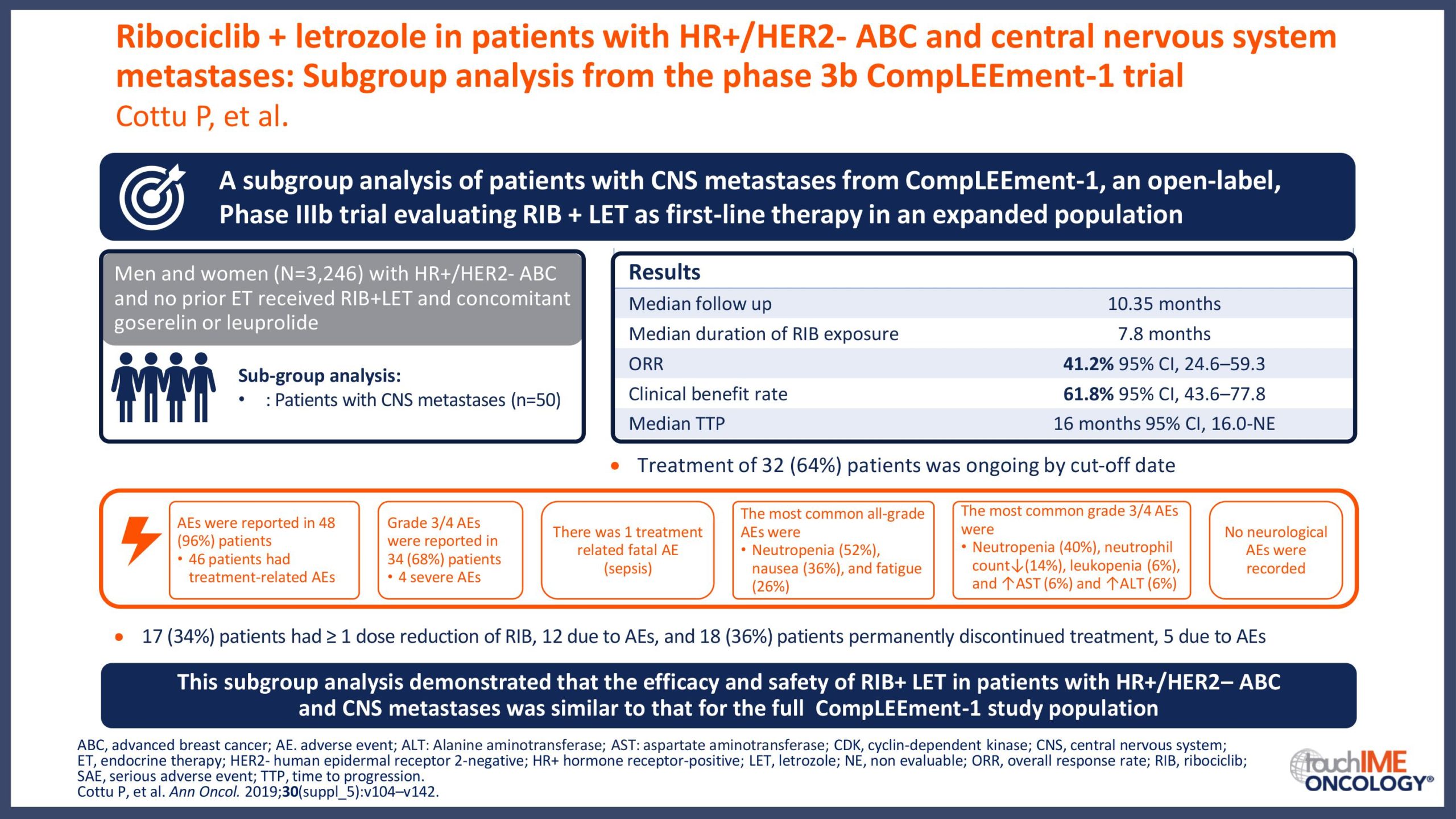
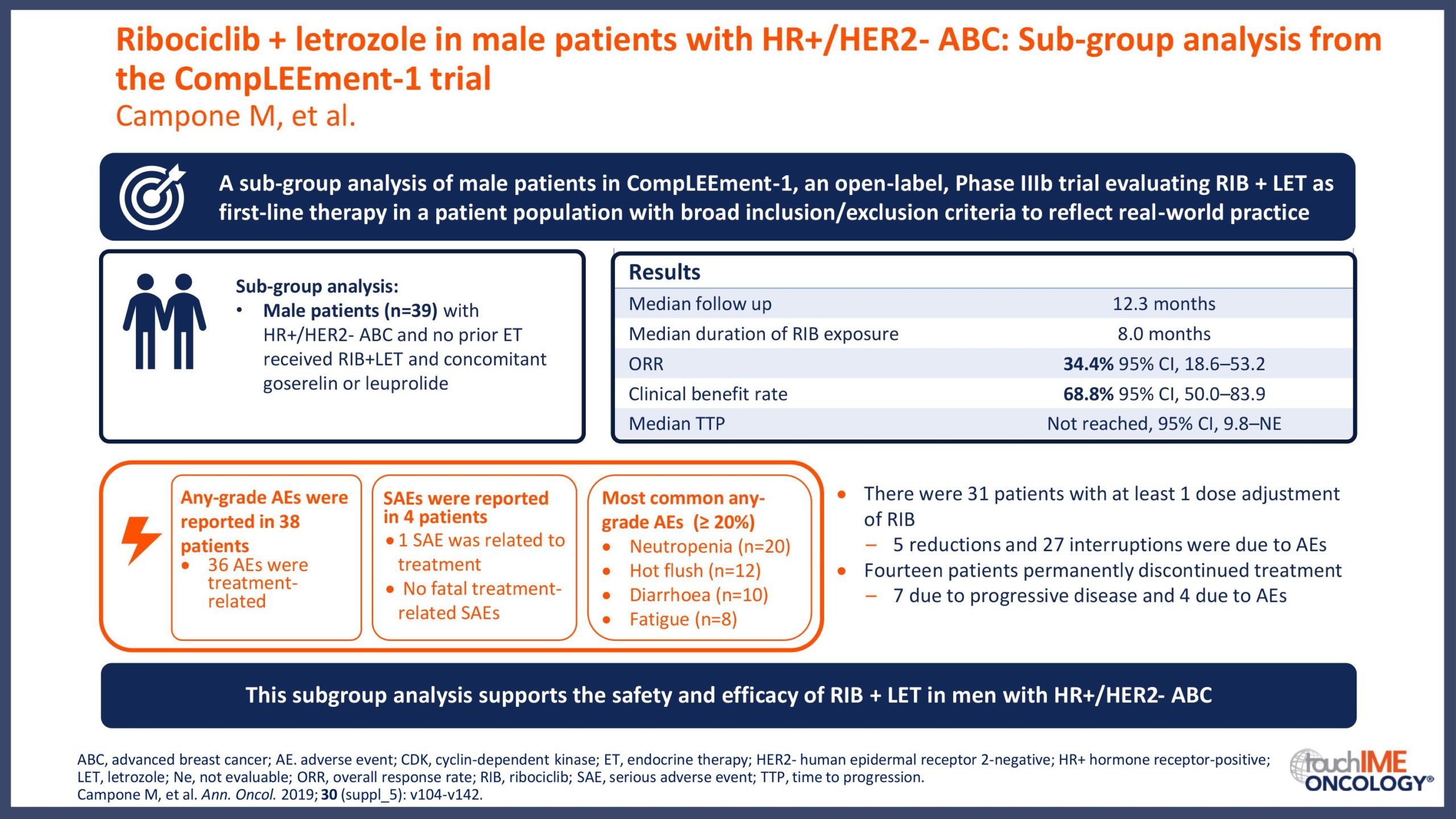
ESMO Congress 2019 – Can patient or disease characteristics predict responsiveness to CDK4/6 inhibitors?
ESMO Congress 2019 – The expanding armamentarium of therapies for advanced breast cancer
Overview
Watch Olivier Trédan consider the impact of emerging data presented at the ESMO Congress 2019 and discuss how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer, including:
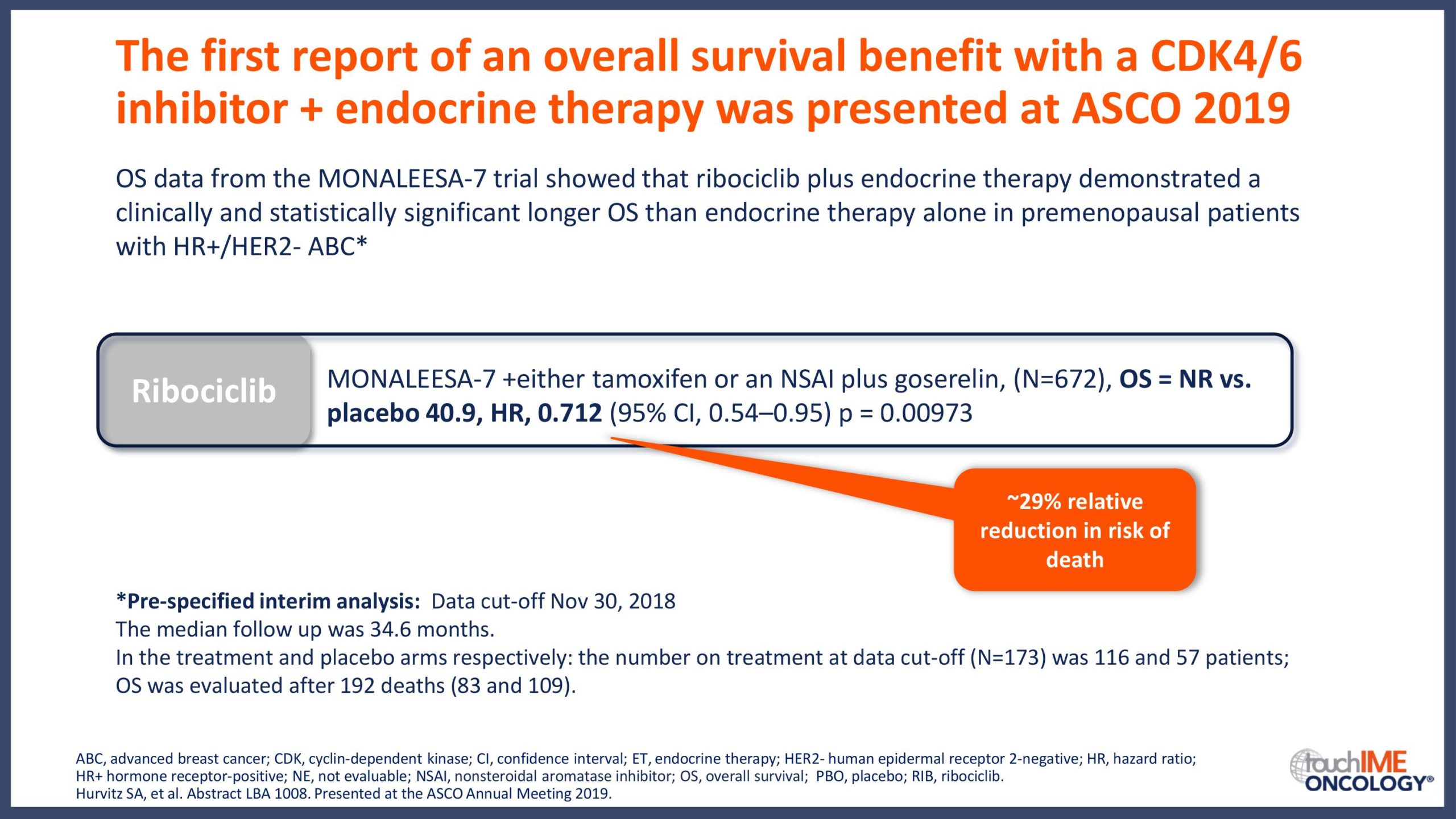
- What are the latest data for CDK4/6 inhibitors in patients with HR+/HER2- advanced breast cancer? Focus on overall survival data and potential implications for optimizing patient outcomes
- Can responsiveness to treatment with CDK4/6 inhibitors be predicted in patients with HR+/HER2- advanced breast cancer? Focus on clinical factors with potential prognostic value
- How is the treatment paradigm for advanced breast cancer evolving? Focus on the expanding armamentarium of therapies
Dr Olivier Trédan is Medical Oncologist at the Centre Léon Bérard (Lyon, France) and Senior Researcher at Lyon University and was the Coordinator of the Teaching and Training Programme there for 5 years. He is currently the Head of the Department of Medical Oncology at the Centre Leon Bérard and leads the breast/gynaecological translational research programme. His clinical practice is devoted to breast and gynaecological cancer patients.
He is the Principal Investigator of several Phase I/II clinical trials investigating molecular screening and new targeted agents, especially for triple-negative breast cancers. Dr Trédan is the author of more than 100 publications, including publications as first or last author in Lancet Oncology, Annals of Oncology, and Journal of the National Cancer Institute.
Dr Oliver Trédan discloses: Advisory roles for Bristol Myers Squibb, Eli Lilly, MSD, Novartis, Pfizer and Roche.
Dr Judy King, Consultant Medical Oncologist at the Royal Free London NHS Foundation Trust, UK, considers the impact of emerging data presented at the ESMO Congress 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Dr Emilio Alba, Director of the Medical Oncology Units at the Hospital Universitario Virgen de la Victoria, Malaga, Spain, considers the impact of emerging data presented at the ESMO Congress 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Masahiro Takada, Assistant Professor at the Department of Breast Surgery, Kyoto University Hospital, Kyoto, Japan, considers the impact of emerging data presented at the ESMO Congress 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Dr Olivier Trédan, Head of the Oncology Department at the Centre Léon-Bérard, Lyon, France, considers the impact of emerging data presented at the ESMO Congress 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Prof. Valentina Guarneri, Associate Professor of Oncology at the Department of Surgery, Oncology and Gastroenterology, University of Padua and Istituto Oncologico Veneto IRCCS, Italy, considers the impact of emerging data presented at the ESMO Congress 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer
Wolfgang Janni, Director of the Department of Obstetrics and Gynaecology of the University of Ulm, Germany, considers the impact of emerging data presented at the ESMO Congress 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Please Select A Video:
Overview & Learning Objectives
Overview
Hear expert review and interpretation of the latest findings for treating HR+/HER2- advanced breast cancer with CDK4/6 inhibitors in this webinar from the European Society for Medical Oncology (ESMO) Congress in Barcelona, Spain, 27 September–1 October 2019.
The information in this activity is intended for oncologists, nurses, and other healthcare professionals involved in the treatment of patients with breast cancer.
Learning Objectives
After participating in this learning activity, you should be able to:
- Describe the role of CDK4/6 inhibitors in the context of the current and evolving treatment landscape for patients with HR+/HER2- advanced breast cancer
- Evaluate the importance of selecting the optimal treatment based on the individual patient, and the challenges around subsequent sequencing of therapy
- Summarize the importance of managing the safety profiles of CDK4/6 inhibitor therapy, and recognize the significance of the multidisciplinary team in optimizing patient outcomes and maintaining on-treatment benefits

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights