touchCONGRESS PARP inhibitors as first-line maintenance therapy in ovarian cancer: How can data from ESGO 2022 guide clinical practice?
Watch this two-part activity exploring the latest data on the use of PARP inhibitors in first-line maintenance therapy in ovarian cancer. Filmed following the ESGO European Congress on Gynaecological Oncology 2022.
Part 1: Watch gynaecological cancer expert Prof. Jonathan Ledermann review key data from ESGO 2022 Watch Now
Part 2: Watch leading experts consider what these data may mean for global and regional practice Select An Interview
Introduction
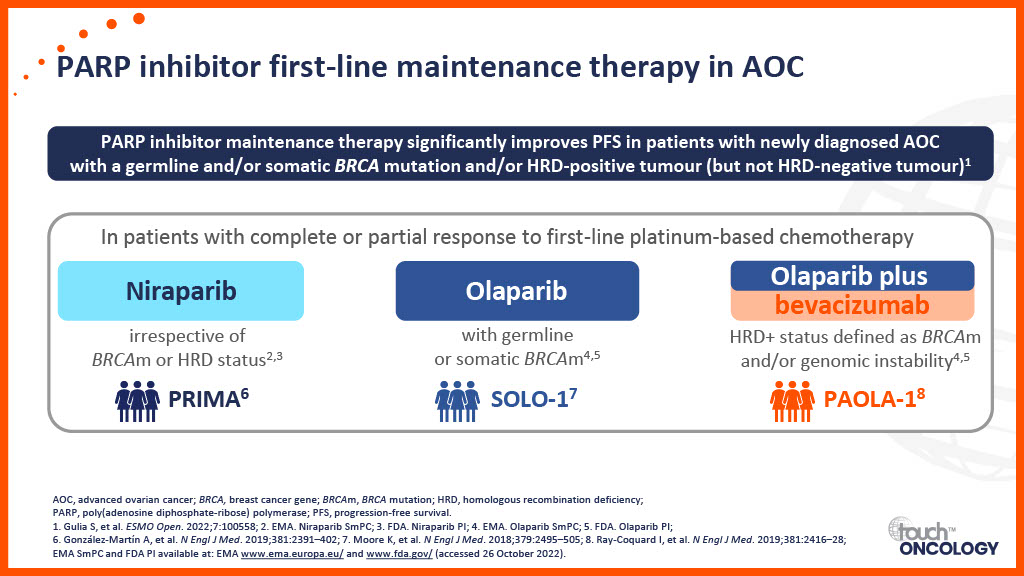
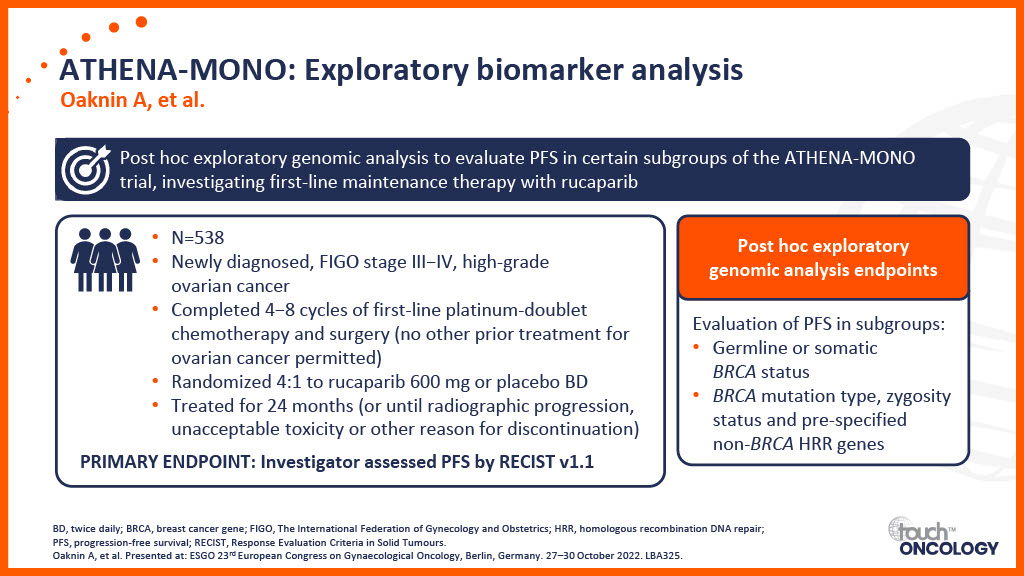
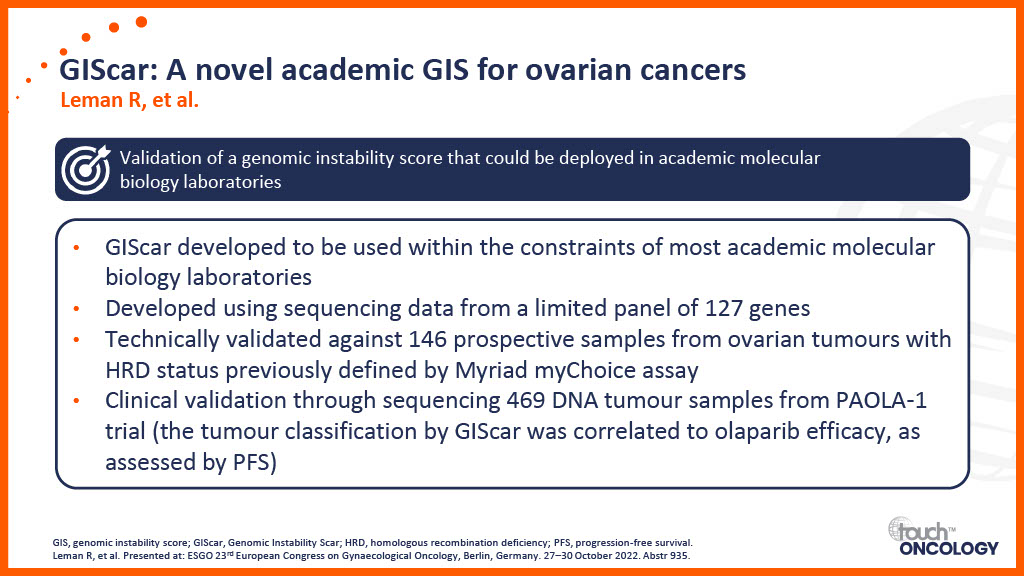
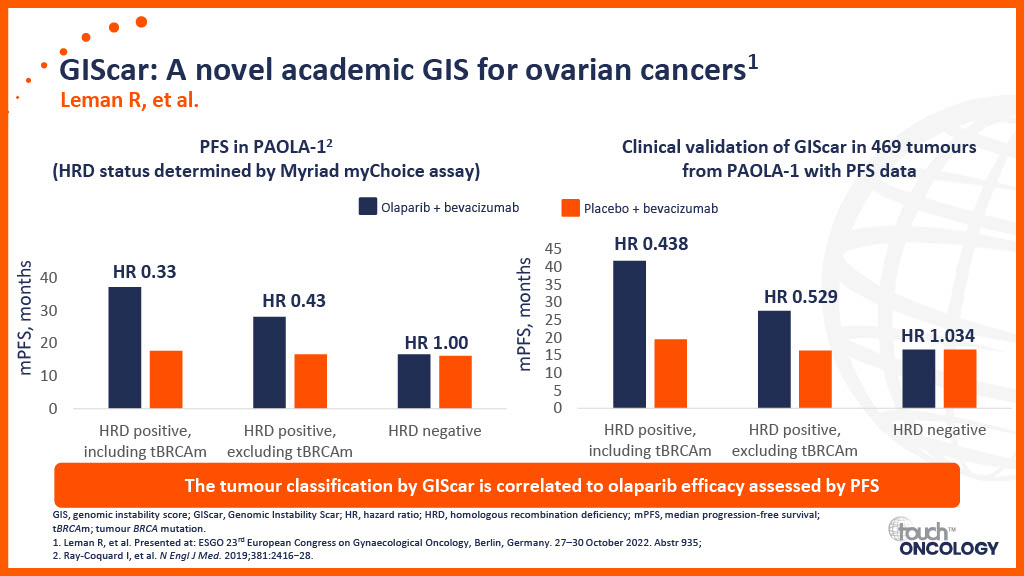
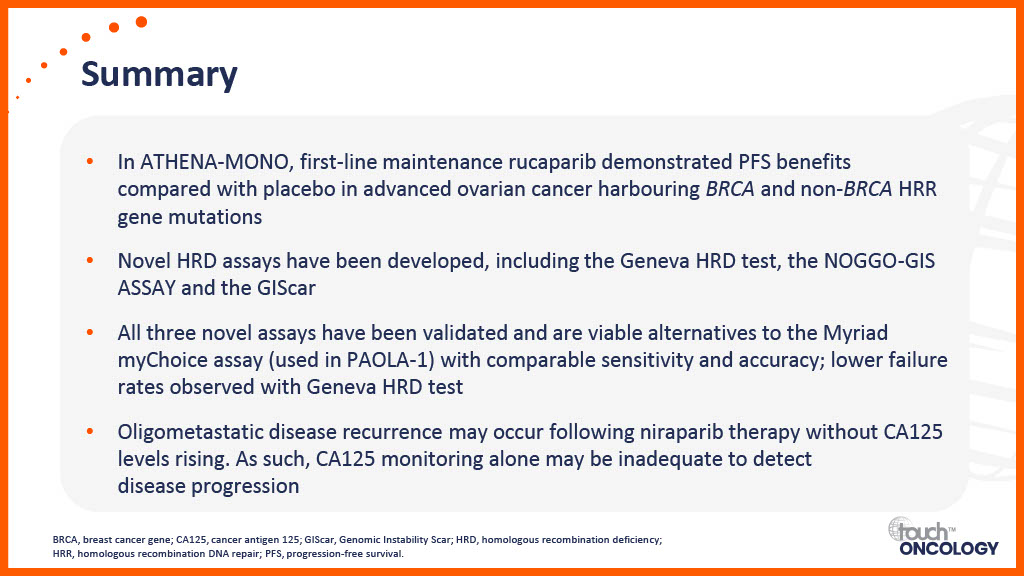
Clinical trial data for PARP inhibitors in the first-line maintenance setting
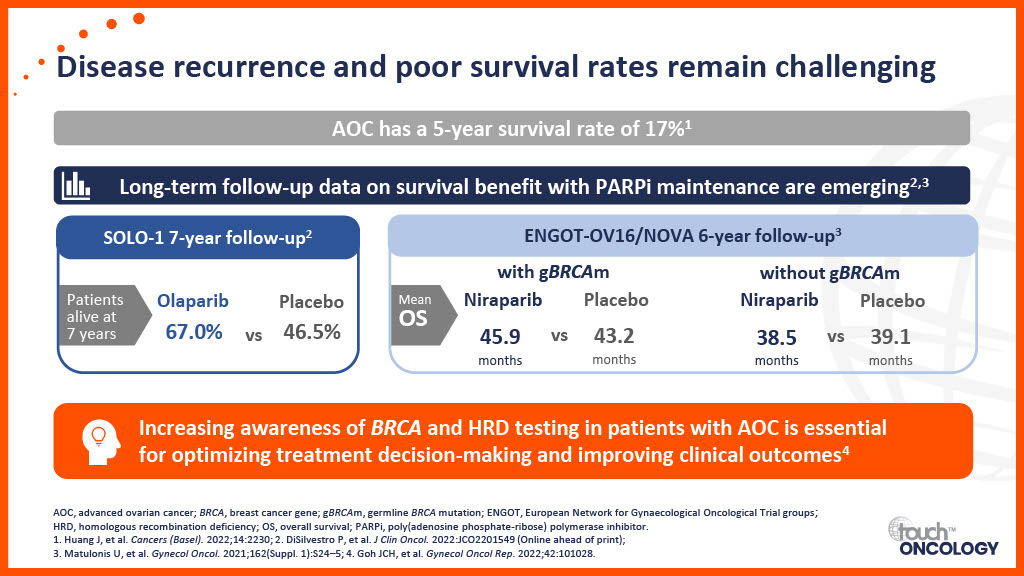
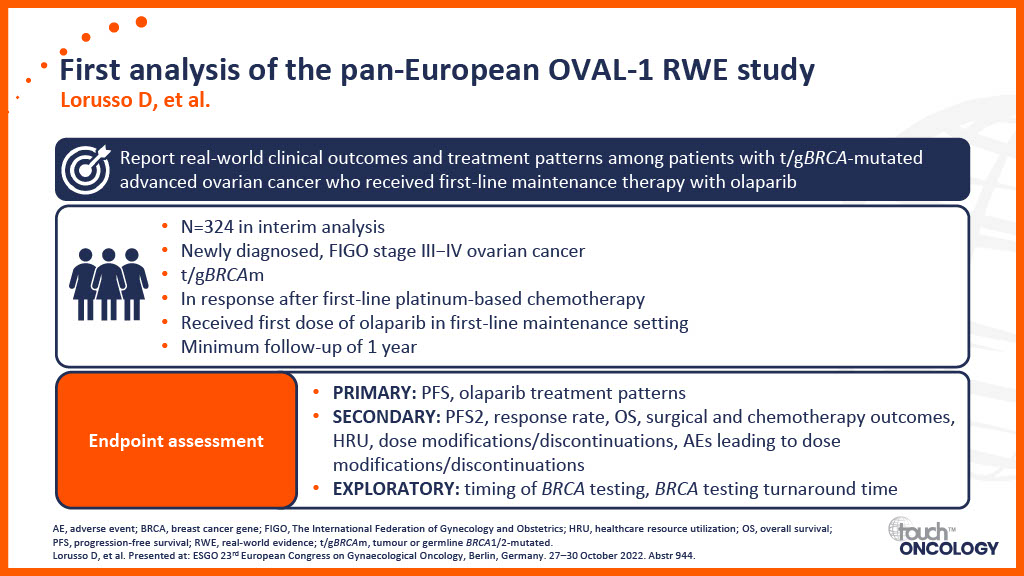
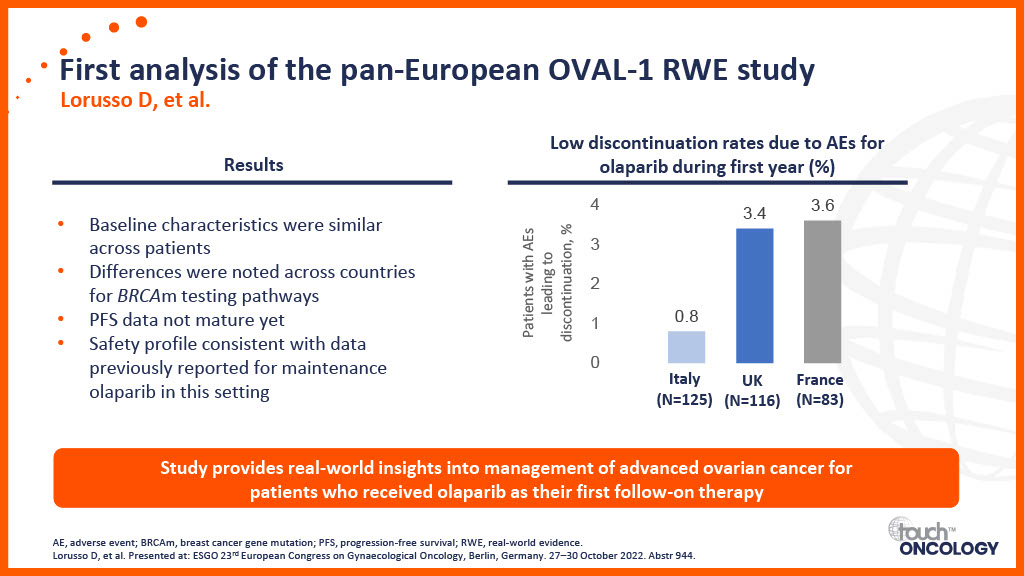
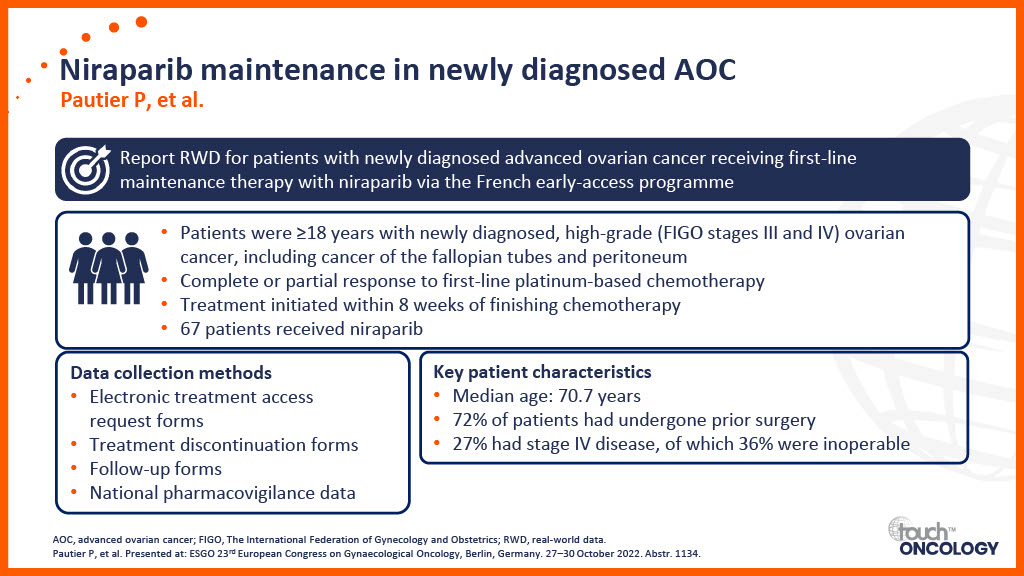
Real-world data for PARP inhibitors in the first-line maintenance setting
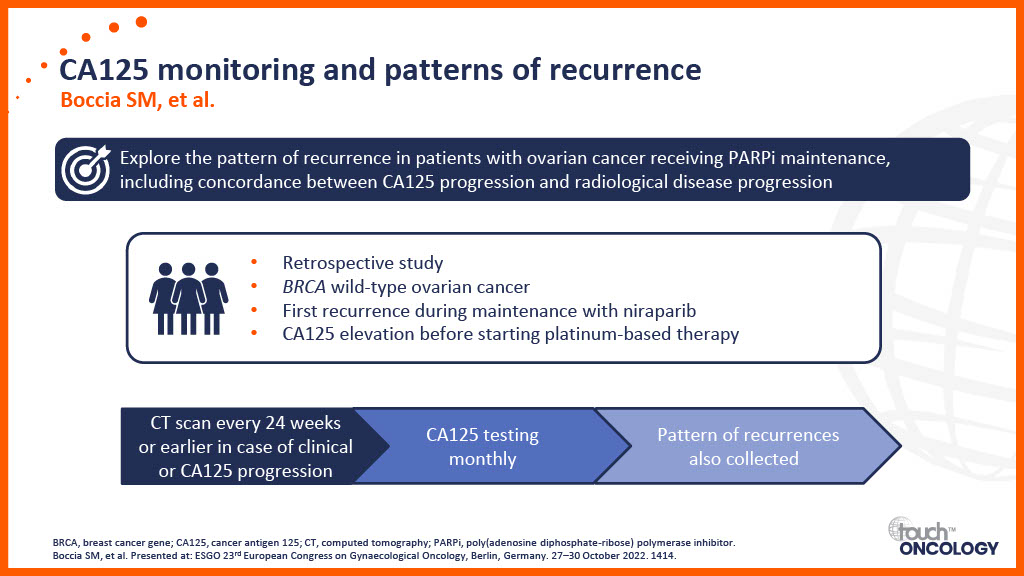
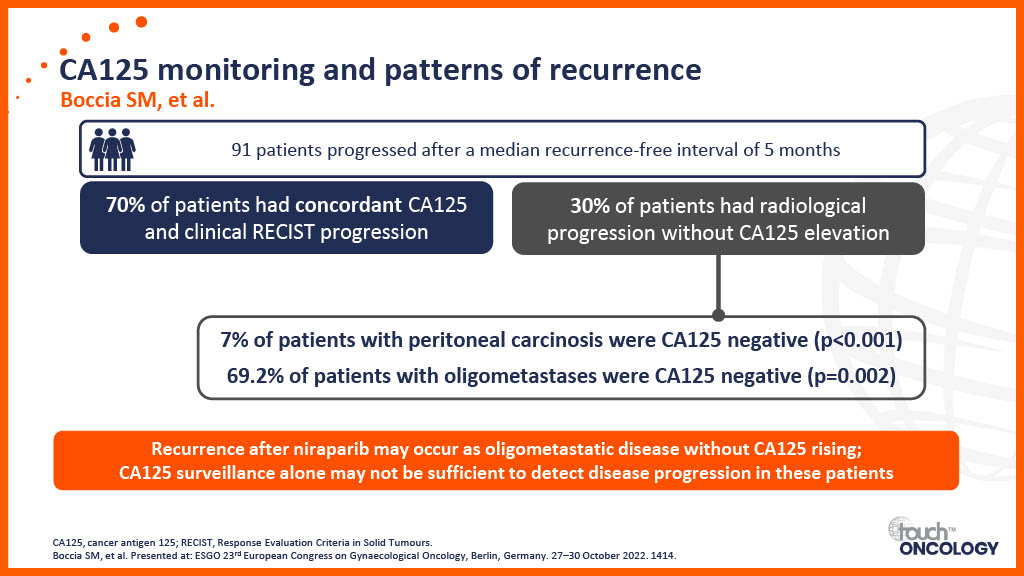
Patient-related considerations for PARP inhibitors in the first-line maintenance setting
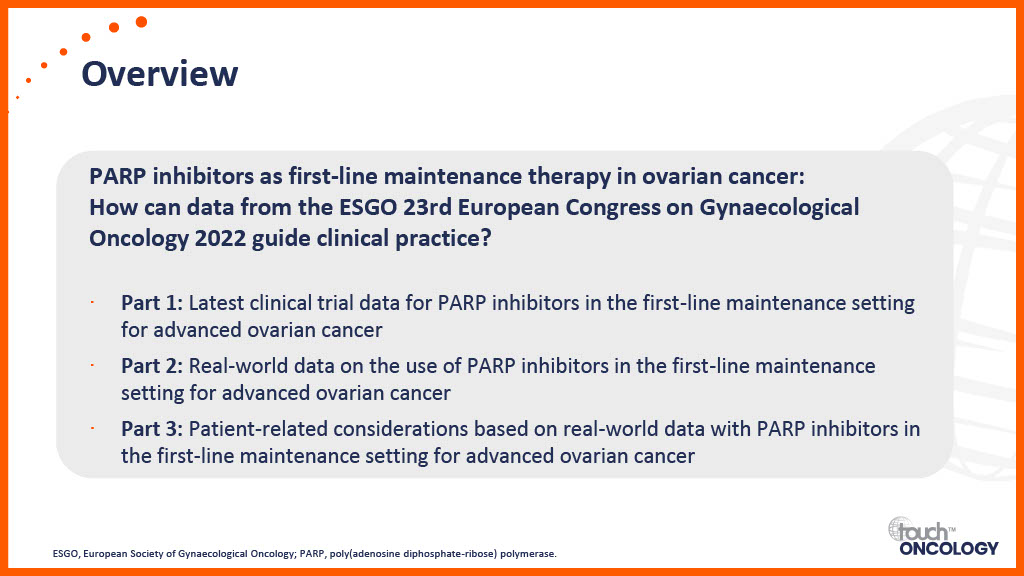
Overview
Watch Prof. Jonathan Ledermann summarize and share his interpretation of the latest data from ESGO 2022 on the use of PARP inhibitors in the first-line maintenance setting in advanced ovarian cancer. During the presentation, he considers:
- The latest clinical trial data relating to PARP inhibitors as a first-line maintenance treatment
- Real-world data on the use of PARP inhibitors as a maintenance treatment
- How the data presented at the congress may impact clinical practice
Prof. Jonathan Ledermann is a professor of medical oncology and clinical director at UCL Cancer Institute, University College London, and a consultant medical oncologist at UCL Hospitals, London, UK. read more
Prof. Ledermann specializes in the treatment of gynaecological cancers and has led several national and international trials in ovarian cancer during 22 years as director of the Cancer Research UK & UCL Cancer Trials Centre. He is a senior investigator for the NIHR (National Institute for Health and Care Research) and a fellow of the Academy of Medical Sciences and editor for the ESMO (European Society for Medical Oncology) clinical practice guidelines on gynaecological cancers.
Prof. Ledermann is the past vice president of ESGO (European Society of Gynaecological Oncology) and has previously been chair of the NCRI (National Cancer Research Institute) UK Gynaecological Cancer Trials Group, chair of the Rare Tumour Group of the CICG (Gynecological Cancer InterGroup) and council member of BGCS (British Gynaecological Cancer Society) and IGCS (International Gynaecological Cancer Society).
Prof. Ledermann serves on editorial boards of several scientific journals and has published widely in the areas of clinical trials in gynaecological cancers, experimental therapeutics, guidelines, and education in gynaecological malignancies.
Prof. Jonathan Ledermann discloses: Advisory board and speaker fees from Artios Pharma, AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Eisai, Ellipses, GlaxoSmithKline, Merck/Merck Sharp & Dohme, Nuvation, Pfizer and VBL Therapeutics. Independent data monitoring committee roles for Mersana and Regeneron. Research grant (to university) from AstraZeneca and Merck/Merck Sharp & Dohme. Clinical trials (to hospital) for AstraZeneca, Clovis Oncology, Eisai, GlaxoSmithKline, Merck/Merck Sharp & Dohme and Pfizer. Non-financial interests with European Society of Gynaecological Oncology (leadership role 2019–2021); Other interests with SAGE Publishing (associate editor: Therapeutic Advances in Medical Oncology).
Please Select A Video:
Overview & Learning Objectives
Overview
Stay up to date with the latest data on the use of PARP inhibitors as first-line maintenance treatment for ovarian cancer in this two-part activity, filmed following the ESGO European Congress on Gynaecological Oncology 2022.
Learning Objectives
After watching this activity, participants should be better able to:
- Outline the latest clinical trial data for PARP inhibitors in the first-line maintenance setting for advanced ovarian cancer
- Describe real-world data on the use of PARP inhibitors in the first-line maintenance setting for advanced ovarian cancer
- Discuss how the clinical trial and real-world data may impact the use of PARP inhibitors in clinical practice

REGISTER NOW FOR FREE ACCESS TO
- 1000+ topical and insightful peer-reviewed journal articles
- 100+ hours of bite-sized congress highlights
- 8 major therapy areas packed with the latest scientific advances
- 150+ specialties offering learn-on-the-go medical education
- + Concise email updates and newsletters so you never miss out