Autologous haematopoietic stem-cell transplantation (HSCT) is widely employed in haematological malignancies including multiple myeloma (MM),1 Hodgkin and non-Hodgkin lymphoma (HL and NHL)2–5 and acute myeloid leukaemia (AML).6,7 High-dose chemotherapy is an effective treatment strategy in numerous malignant conditions, however, it requires the subsequent use of autologous HSCT in order to restore bone marrow function, mostly using HSCs from the patient’s peripheral blood.8 Rates of autologous HSCT have increased steadily during the past 2 decades.9–12 In 2014, more than 40,000 HSCT (57% autologous) were performed in Europe.13 The main indications for HSCT were leukaemias (33%; 4% autologous); lymphoid neoplasias (57%; 89% autologous); solid tumours; (4%; 97% autologous) and non-malignant disorders; (6%; 12% autologous).13 Recent trends in transplant activity include increased use of allogeneic HSCT for AML in first complete remission, myeloproliferative neoplasm (MPN) and aplastic anaemia with decreasing use in chronic lymphocytic leukaemia (CLL); and increased autologous HSCT for plasma cell disorders.13 The ability to improve patient outcomes with autologous HSCT is directly dependent, however, on successful mobilisation and collection of stem cells.
Various advances in HSCT over the past decade, including new stem cell mobilisation techniques, have led to the need to reassess strategies to optimise outcomes. In October 2015, clinicians from Europe, North America and Asia compared their experiences and discussed these issues at a Sanofi-sponsored satellite workshop at the 3rd International Congress on Controversies in Stem Cell Transplantation and Cellular Therapies (COSTEM 2015). This review aims to discuss the challenges of finding the optimal mobilisation strategy in the context of these discussions.
Key stages of haematopoietic stem-cell transplantation The HSCT process can be summarised as follows: administration of mobilisation agents, mobilisation, collection by leukapheresis, preparation of product for storage, cryopreservation, administration of high-dose chemotherapy, stem cell transplantation, and engraftment and recovery.14 HSCs usually circulate in small numbers in peripheral blood, therefore, their mobilisation from bone marrow into peripheral blood following treatment with chemotherapy and/or cytokines is an essential part of HSCT, and is one of the major challenges of the process.15
Progenitor stem cells express the cell surface marker antigen CD34, which is used in clinical practice to determine the extent and efficiency of peripheral blood stem cell collection.16 The number of peripheral blood CD34+ cells is used to monitor the timing of leukapheresis for autologous transplantation.17 Before collection, the number of CD34+ cells should ideally exceed 10-20/μl in peripheral blood.18
In terms of transplantation, a number of Phase II studies have established a correlation between CD34+ dose and outcome in terms of progressionfree survival (PFS) and overall survival (OS).19 Most clinical centres regard 2.5–4 x 106 CD34+ cells/kg body weight as an adequate cell number for autologous HSCT and 2.0 x 106 CD34+ cells/kg as the absolute minimum; this is based on a substantial body of clinical data.18,20–24 However, a minority of experts recommend increasing this threshold. Some studies suggest that doses exceeding 5 x 106 cells/kg are necessary for optimal engraftment23,25 and to reduce febrile complications and antibiotic use after transplantation.26 A 2000 literature review concluded that a of ≥8 x 106 CD34+ cells/kg is optimal, and correlated cell dose to platelet recovery,25 but this has been disputed. In addition, high levels of circulating CD34+ cells have been associated with better outcomes in MM27 and NHL.28 The reported improvement in outcomes may be due to decreases in non-relapse mortality from improved haematologic reconstitution and lower rates of infection.
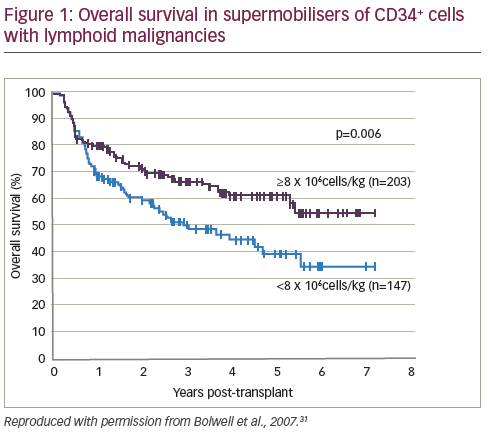
Conversely, some studies have concluded that high cell doses are not correlated with improved outcomes. A study of patients with MM and NHL found that cell dose did not affect OS at one year.29 A cohort study (n=80) demonstrated that high dose CD34+ cells were not associated with lower blood component consumption after HSCT.30 In a retrospective study, patients (n=350) who mobilised high numbers of CD34+ cells (so-called supermobilisers) had improved outcomes in autologous HSCT for NHL and HL (see Figure 1).31 However, a similar study design (n=39) of patients with MM or Waldenström macroglobulinemia (WM) found no correlation between survival and number of mobilised CD34+ cells.32
In summary, there are insufficient data to conclude that high cell numbers are necessary in autologous HSCT. The optimum dose has not been comprehensively evaluated in prospective studies, most of which are registry-based and retrospective.
What is the outcome of mobilisation?
Stem cell collection requires mobilisation of the HSCs, which aims to obtain as many HSCs of the best possible quality, in the first mobilisation attempt, and preferably a single leukapheresis session.23 Failure to mobilise a sufficient number of CD34+ cells may result in ineligibility for transplantation and subsequent relapse or the need for multiple leukapheresis sessions, adding to the cost and inconvenience to the patient. Ultimately, a bone marrow harvest may be needed.
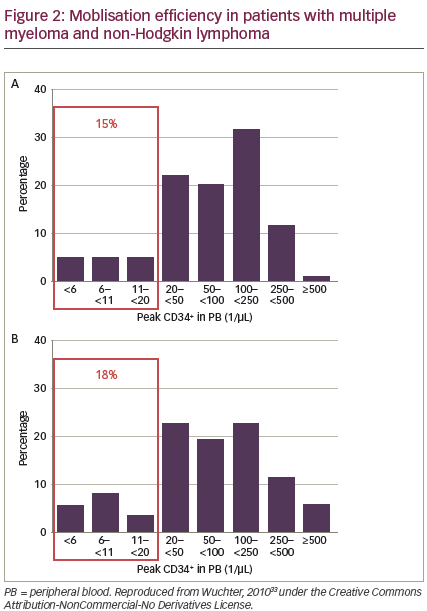
Patients undergoing autologous HSCT differ in their ability to mobilise cells.33 HSC mobilisation may be affected by: age, ethnicity, type and dose of cytokines used, the patient‘s diagnosis, number and type of previous chemotherapy cycles or radiation, and interval from last chemotherapy cycle.33–40 However, these findings are not consistent across all studies and it is difficult to predict how individual patients will respond. Peripheral blood CD34+ cell counts correlate with numbers of CD34+ cells collected.33 A significant minority of patients receiving standard mobilisation fail to mobilise enough CD34+ cells. In a 2010 study, before the availability of plerixafor, 15% and 18% of patients with MM and NHL, respectively, were considered ‘poor mobilisers’. However, two-thirds of these patients were finally able to receive autologous HSCT (see Figure 2).33
Several mobilisation strategies may be employed (see Figure 3).36 Mobilisation with granulocyte-colony stimulating factor (G-CSF) alone gives a predictable peak CD34+ level within 4–5 days, allowing for reliable apheresis scheduling18,23 Notably, it is also associated with a relatively short window of opportunity for successful leukapheresis of 1–3 days.18,23,24
Another method to mobilise HSCs involves the administration of chemotherapy, usually a cyclophosphamide-containing regimen that may be given in conjunction with G-CSF.41 In a 2007 study of 175 lymphoma patients undergoing autologous HSCT, those with successful G-CSF mobilisation had quicker platelet recovery and improved PFS and OS compared with patients who had adequate collection only after chemotherapy mobilisation or those who failed to collect an adequate graft with either type of mobilisation.42 However, using G-CSF alone is cheaper, more convenient and associated with fewer adverse effects
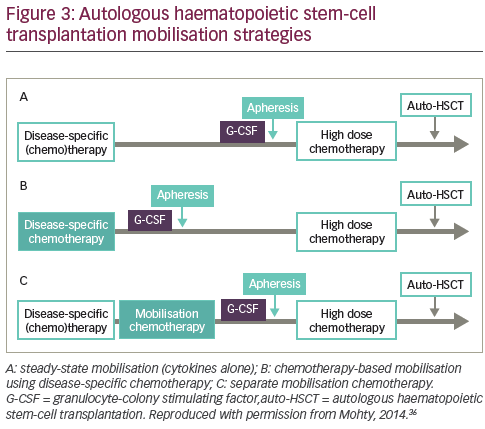
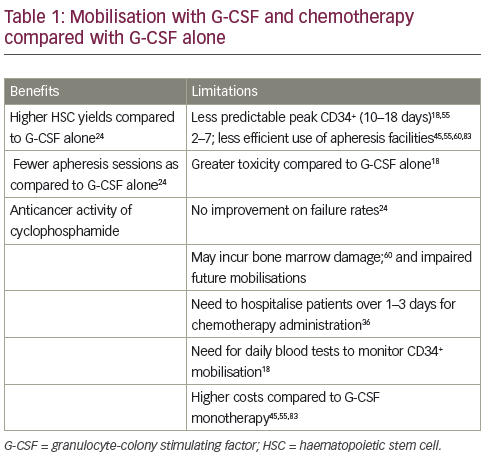
than chemotherapy plus G-CSF.36,43–46 The benefits and limitations of adding chemotherapy are summarised in Table 1. A 1997 study (n=40) found that chemotherapy plus G-CSF was not superior to G-CSF alone in terms of HSC yields.47 In addition, a 2015 study (n=167) concluded that mobilisation with high-dose cyclophosphamide before autologous HSCT increased toxicity without positively impacting long-term outcomes in MM.48 However, other studies have demonstrated enhanced HSC collection after chemomobilisation.24,49 Furthermore, one study suggested that the use of chemotherapy may minimise tumour contamination of the HSC product.50 Chemotherapy can, however, impair future mobilisations and make the timing of the circulating CD34+ cell peak less predictable, so patients may require apheresis at the weekend.51 In summary, no large studies to date have conclusively established a difference between chemotherapy and cytokine-only mobilisation in the amount of tumour contamination of the stem cell product and transplantation outcomes, such as engraftment and survival.
Plerixafor is a novel CXCR4 chemokine-receptor antagonist used for autologous HSCT mobilisation. Plerixafor is a bicyclam molecule that inhibits the stromal cell-derived factor 1 (SDF-1) alpha/CXCR4 binding that occurs between CD34+ stem cells and the bone marrow stroma, causing the release of CD34+ stem cells into the peripheral blood.52 It gained Food and Drug Administration (FDA) approval in 2008 and European Medicines Agency (EMEA) approval in 2009. It is indicated in combination with G-CSF to enhance mobilisation of HSCs to the peripheral blood for collection and subsequent autologous transplantation in adult patients with lymphoma and MM whose cells mobilise poorly.44 It is also recommended in patients over 60 years old and/or prior myelosuppressive chemotherapy and/or extensive prior chemotherapy and/or a peak circulating stem cell count of less than 20 stem cells/microliter, or those who have been identified as predictors of poor mobilisation. The recommended dose of plerixafor is 0.24 mg/kg body weight/day. It should be administered by subcutaneous injection 6–11 hours prior to initiation of each apheresis following a 4-day pre-treatment with G-CSF. Common side effects include diarrhoea, nausea, as well as injection and infusion site reactions.44 The efficacy of plerixafor for autologous HSC mobilisation has been demonstrated in a number of clinical studies in haematological malignancies,53–55 including two Phase III clinical trials in patients with NHL or MM.56,57
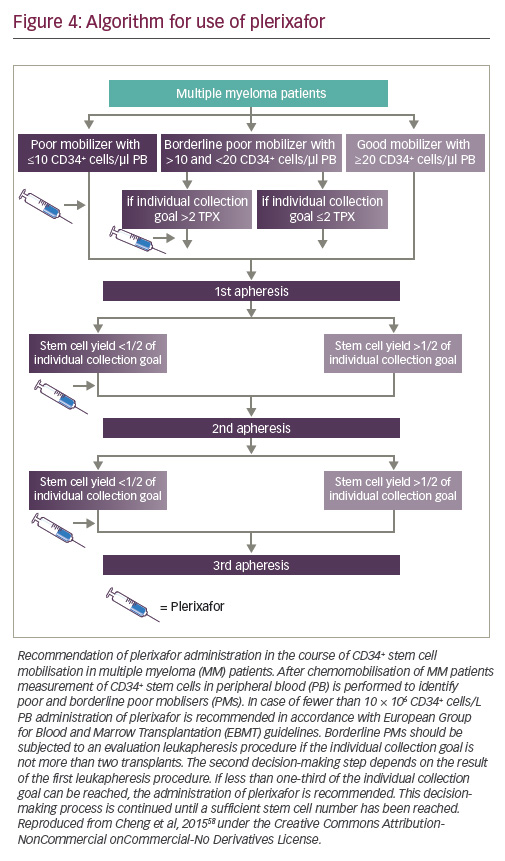
Plerixafor can be given either pre-emptively, typically in patients with peripheral blood counts of <10 CD34+ cells/μl, or as a rescue strategy
in poor mobilisers if the cell yield is less than one-third of the individual collection goal, to avoid the need for multiple leukapheresis sessions.58,59 It is generally considered that not more than three consecutive leukapheresis sessions should be performed for collecting one transplant. An algorithm based on a single centre database is shown in Figure 4.58
A retrospective analysis of 1,834 patients who underwent stem cell mobilisation for autologous transplantation from November 1995 to October 2006, found that those receiving G-CSF plus plerixafor had the lowest failure rates (p=0.03). NHL patients remobilised with G-CSF who waited ≥25 days before remobilisation had lower CD34+ cell yield than those who waited ≤16 days (p=0.023).24 Plerixafor ‘on demand’ after chemotherapy plus G-CSF is an effective first-line mobilisation strategy with myeloma and lymphoma with delayed haematopoietic recovery and <10/μL CD34+ cells, but the timing of administration and criteria for patient selection remain to be established.60 A retrospective study (n=66) found that plerixafor/G-CSF and cyclophosphamide/GCSF for upfront mobilisation of CD34+ cells yielded similar numbers of cells collected, costs of mobilisation, and clinical outcomes. In addition, plerixafor/G-CSF mobilisation was associated with predictable days of collection, no weekend apheresis procedures and no unscheduled hospital admissions.45
The timing of leukapheresis after plerixafor injection is important in very poor mobilisers: The recommended timing for plerixafor administration is between 6 and 11 hours before leukapheresis, but this approach has been logistically difficult. In clinical practice, the interval may depend on whether treatment was given on an inpatient or outpatient basis. Studies have compared the efficacy at different time intervals.61 In a good mobiliser the interval between plerixafor injection and leukapheresis can be wide, when the reason for plerixafor is to obtain high numbers of cells. Conversely, some experts believe that plerixafor has a short interval of action, so the interval between administration and initiation of leukapheresis may be reduced for poor mobilisers who will only have very few CD34+ cells. Few data are available on plerixafor kinetics in patients with very poor CD34+ cell mobilisation. In one series of 11 patients, peripheral CD34+ cell counts fell after 3 hours. The authors concluded that following the recommended schedule, leukapheresis at the conventional 11 hours after plerixafor injection would have failed to obtain enough cells for transplantation.62
Aiming to perform all leukapheresis sessions on weekdays, when laboratory staff are available, can limit the number of patients receiving apheresis because of the limited availability of apheresis machines. The optimal days for planning the initial leukapheresis session are Monday to Wednesday, allowing the opportunity to successfully complete the collection process by Friday, even if one or two additional apheresis sessions are required.
Another potentially useful approach is optimising the leukapheresis strategy. Terumo BCT recently introduced a new system for mononuclear cell (MNC) collection that allows for the continuous collection of MNCs, unlike the original system (Spectra Optia®, Terumo BCT, Colorado, US), which included a chamber for two-step cell separation. However, a comparative study (n=150) of the two apheresis systems in regard to specific performance parameters found that both systems were equally efficient in collecting CD34+ cells.63 In addition, a formula to predict collection of CD34+ cells/kg has been validated. Using this formula, clinicians can adjust leukapheresis duration and blood volume processed, to achieve the patient’s collection target in only one apheresis without spending longer on the machine than necessary. This will theoretically allow for individualisation of collection for any donor once the peripheral blood CD34+ cell count and optimal goal of collection were known.64
The importance of graft composition in HSCT
Until recently, CD34+ stem cell dose has been the accepted measure of graft quality. Attention is now moving towards more detailed aspects of graft composition, such as CD34+ subpopulations. Several studies have found lower rates of relapse, defined as the absolute lymphocyte count on day 15 after HSCT, in patients with rapid lymphocyte recovery.65–71 A higher nucleated cell dose has been associated with increased survival and decreased relapse in patients in second remission or beyond.72 Interestingly, in this study, the number of CD34+ cells did not have any significant influence on the transplant outcome, but the intensity of the preparative regimen was lower in comparison with the conditioning used in the other studies. It should also be noted that these were small studies and their findings are still under debate.
The use of plerixafor affects the graft composition: it appears to mobilise more primitive CD34+/CD38– stem cells compared with G-CSF, as well as higher T- and natural killer (NK)- cells.73–77 Further studies are needed to investigate the impact of this on clinical outcomes.73 In a study (n=58) of patients with B-cell lymphoma or CLL who underwent auto HSCT after myeloablative conditioning, the number of CD34+CD38–HLA-DRcell subsets was associated with faster early engraftment but had no effect on long-term outcomes.78 It has been suggested that the quality of CD34+ cells from low mobilisers may be inferior to that of HSCs from high mobilisers. However, a study found that the proportions of primitive and quiescent CD34+ subsets were comparable across mobilisation groups and concluded that HSC products from low mobilisers are of similar quality to high mobilisers.79
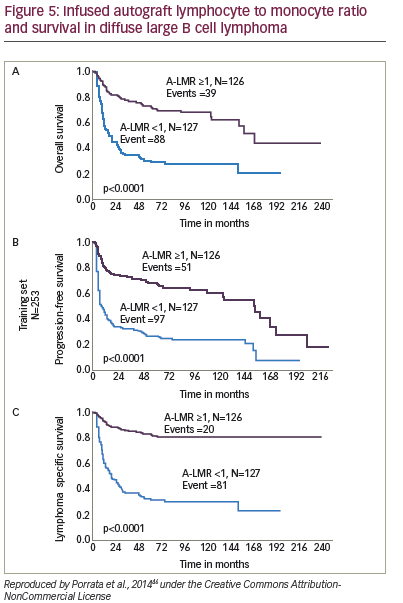
An increasing body of evidence suggests that non-CD34+ cells predict early engraftment and better outcomes. Immune recovery is as crucial as haematopoietic reconstitution, and higher – and NK-cells infused within the graft have been correlated with better outcome in autologous transplants. The NK-cell count at day 15 has been associated with enhanced OS and PFS in a study of patients with MM (n=15) and NHL (n=4).80 Dendritic cells have also been identified as predictors of improved survival in autologous HSCT.81 Furthermore, a study (n=65) of autologous HSCT for haematological malignancies found that the number of CD8+ cells in the graft is important for early lymphocyte recovery.82 Again, further studies are required to confirm these findings. A study (n=190) of NHL patients found that infused peripheral blood autograft absolute lymphocyte count correlated with outcomes at day 15 days.66 A later study found that the lymphocyte to monocyte ratio in the graft was a key predictor of survival in diffuse large B-cell lymphoma (see Figure 5).
Other factors influencing the mobilisation process
The wishes of the patient are important in choosing mobilisation strategies. Patients often undertake considerable online research and may have strong opinions regarding their treatment. New predictive formulas may reduce uncertainty for the patient, for example telling him or her that there is an 80% chance of achieving their collection target in one session. Using the predictive formula can avoid patients undergoing unnecessarily prolonged procedures. Stem cell mobilisation and collection may take place in an outpatient or an inpatient setting.
Summary and concluding remarks
The variation of study findings with respect to optimal CD34+ cell doses and use of mobilisation agents illustrates the challenges in optimising leukapheresis outcomes for HSCT. Many studies to date are not representative of real life situations: most were performed in the 1990s,many were in cancer patients and analysed a variety of disease entities together. Early studies in MM did not use novel induction therapy and few studies compare engraftment kinetics or outcomes in stem cell mobilisation techniques that are currently in use. Prior chemotherapy could also be a confounding factor in comparing patient groups. In a study of autograft lymphocyte to monocyte ratio, patients who had received fewer chemotherapy courses had better prognoses,46 perhaps because they have more treatment-sensitive disease, or due to toxic effects of chemotherapy. Importantly, there are no prospective studies on CD34+ cell doses.
Although a considerable body of evidence suggests that the cell dose influences transplant outcomes in HSCT, the graft composition also appears to play a role. The importance of graft composition needs to be elucidated and several questions remain. For example, does a higher content of T- and NK-cells achieve a quicker immune recovery, which may prevent infections and enhance anti-disease activity? Does the number of immune cells within the apheresis product correlate with survival following HSCT? The potential impact of cyclophosphamide, one of the most commonly used chemotherapy mobilisation regimens, on graft composition also remains unknown. This agent is known to be lymphotoxic and thus reduces the number of T-cells, NK- and B-cells in the graft. By contrast, plerixafor shows increased mobilisation of T- and NK-cells independently of CD34+ cell yield. More prospective study data are needed for a fuller understanding of engraftment and immunologic regeneration using different mobilisation protocols. Tailoring the dose of the different cell subsets contained in the graft to each individual patient might improve transplant outcomes.
In conclusion, many factors influence outcomes in HSCT. Patient factors may have more effect on overall outcome than graft content. Studies aimed at assessing the importance of any factor would need multivariate analysis of a large number of patients.







