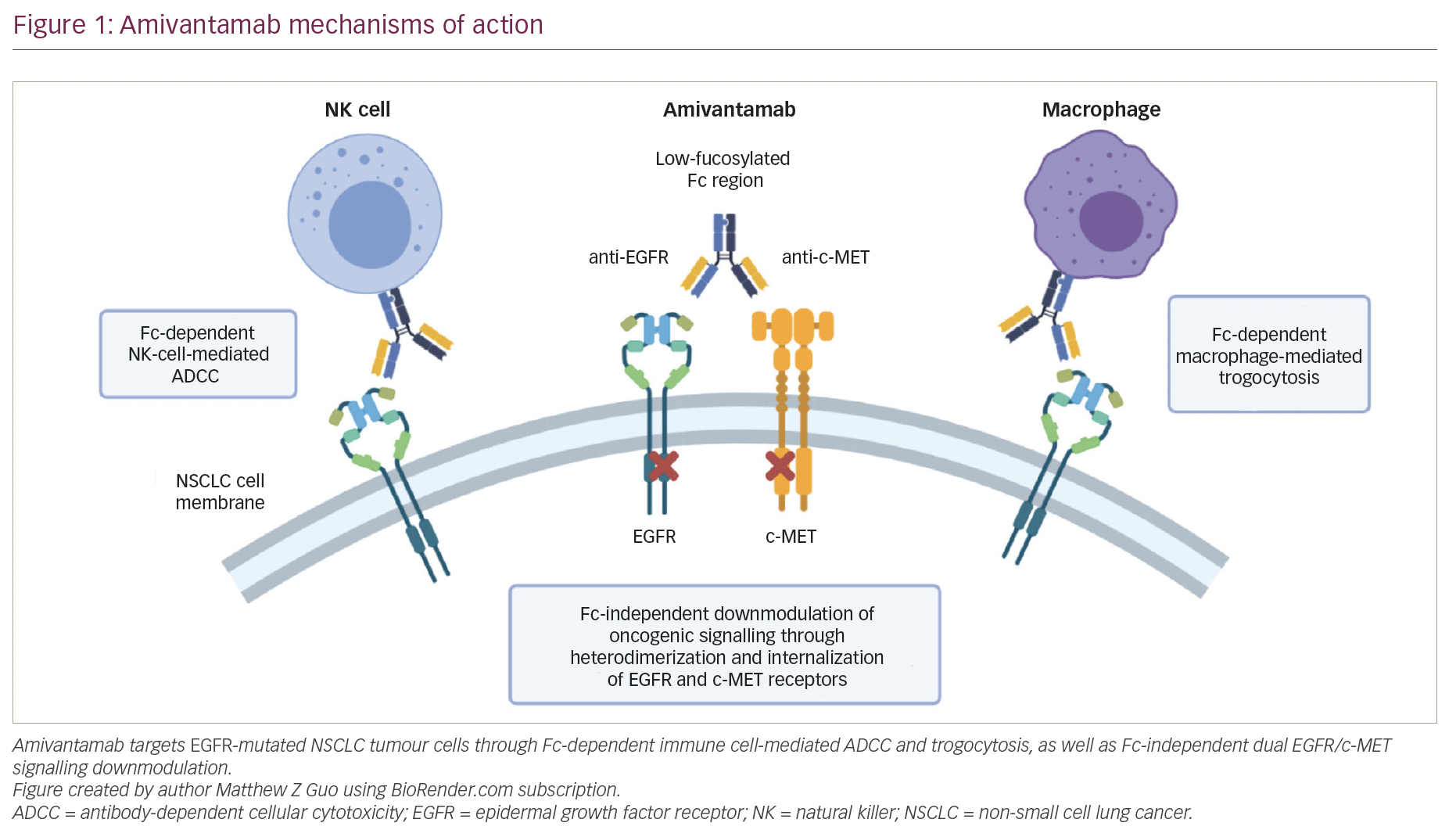
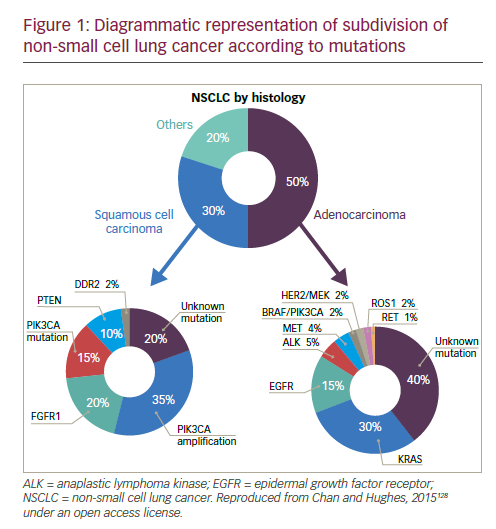
Lung cancer is the leading cause of cancer mortality worldwide1 and remains one of the major therapeutic challenges in oncology. Traditionally, lung cancer is subdivided based on histology: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) have completely different molecular and therapeutic profiles. The most common class is NSCLC, accounting for 85% of all cases.2 The prognosis of NSCLC dramatically changed following the discovery of genetic alterations affecting oncogenes, called drivers, including epidermal growth factor (EGFR) mutations, anaplastic lymphoma kinase (ALK) rearrangement, ROS1 or RET rearrangements, MET mutations or amplification, as well as BRAF or HER2 mutations (see Figure 1). The ability to target tumours at the molecular level has led to a paradigm shift in the management of patients with these mutations.
The availability of new therapies with differing modes of action is potentially confusing, not only for primary care physicians but also for medical oncologists. Furthermore, unmet needs remain in the treatment of NSCLC, including the development of resistance to targeted therapies and the fact that not all molecular subtypes are actionable to date. Furthermore, a large group of patients does not suffer from tumours characterised by oncogenic alterations, with the vast majority deprived of any actionable genetic change. These patients are currently treated by chemotherapy. There is a need for new treatments for these patients, and immunotherapy represents a promising approach, but also confers a challenge: identifying patients who will benefit optimally from these treatments. This article aims to discuss the use of targeted therapies and immunotherapy in the setting of NSCLC.
Targeted therapies for non-small cell lung cancer
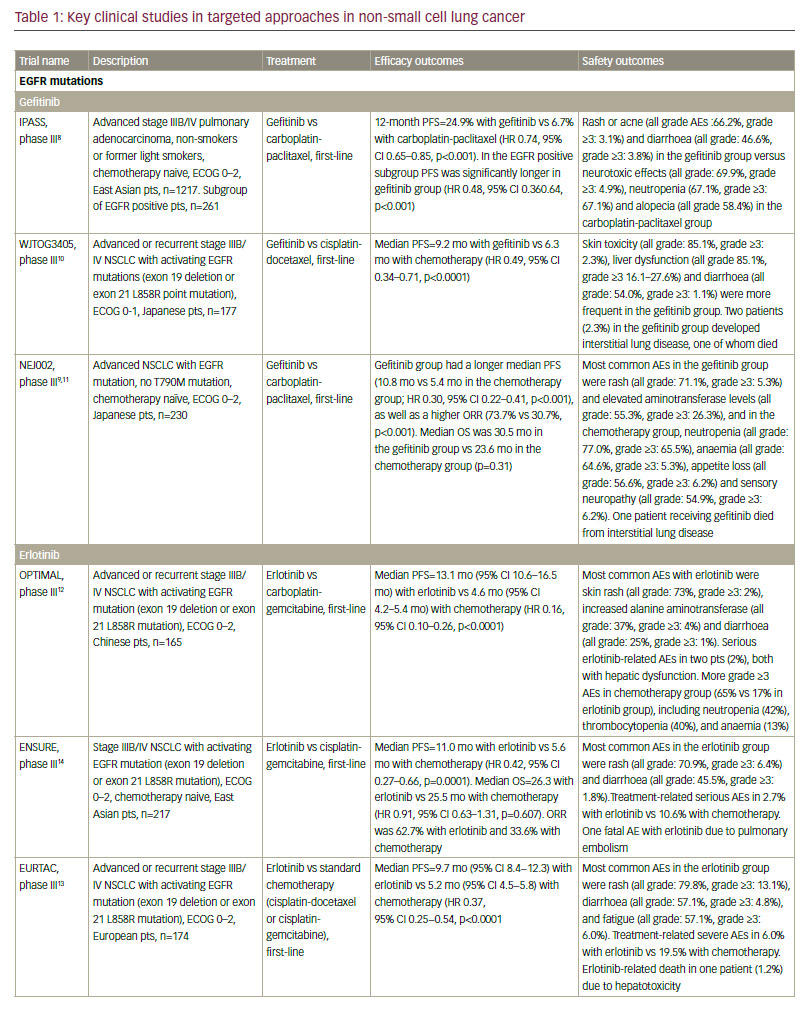
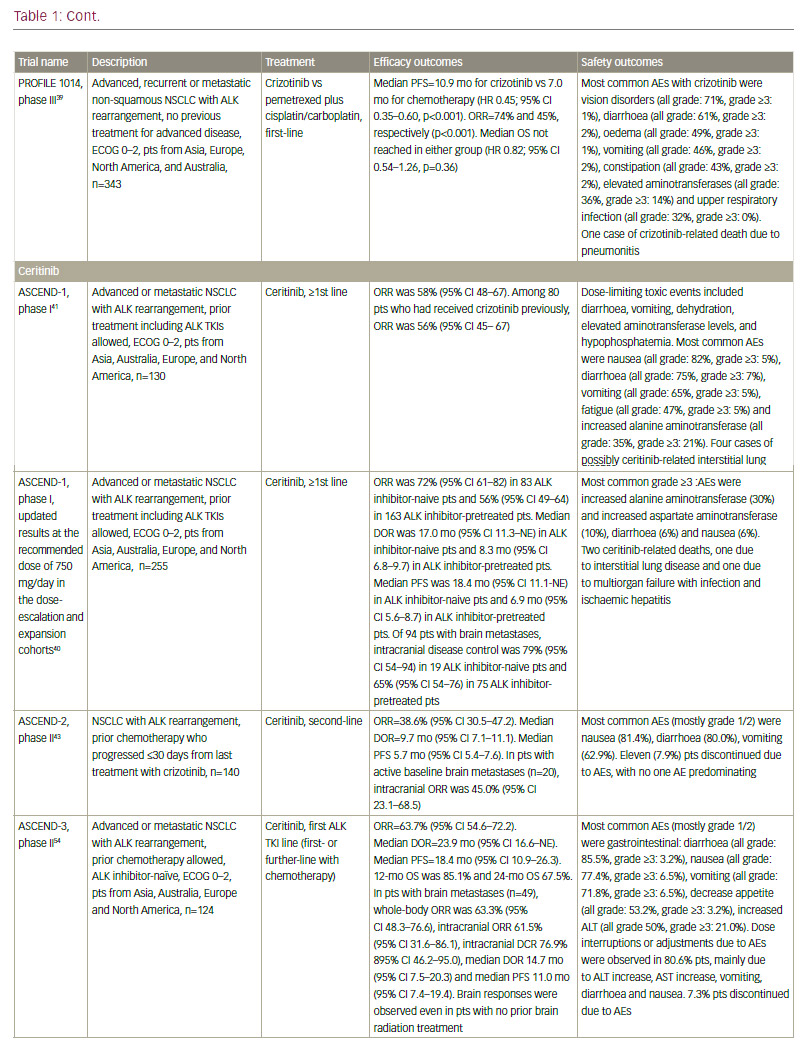
Standard-of-care molecular characterisation of advanced NSCLC is performed by analysis of activating mutations of EGFR (in exons 19 and 21) and detection of an ALK rearrangement.3 Such screening of NSCLC patients for driver mutations have been standardised and validated.4 However, mutations on the EGFR gene are seen in fewer than 10% of NSCLC patients in Western populations,5,6 although this is higher (up to 50%) in Asian populations.5,7 The mutation has been reported with a higher frequency in non-smokers, women, and presence of adenocarcinoma.6 Clinical trials of approved targeted therapies for NSCLC are summarised in Table 1. The use of EGFR tyrosine kinase inhibitors (TKIs) is recommended as first-line therapy
for patients with an EGFR driver mutation, following an accumulation of evidence of superiority compared with chemotherapy. The first EGFR TKI was gefitinib8–11 followed by erlotinib12–14 and the second-generation TKI afatinib.15,16 In addition, dacomitinib is being evaluated as first-line therapy.17 However, despite high response rates, resistance to EGFR inhibitors invariably ensues in the majority of patients. One of the most common mechanisms of EGFR TKI resistance has been attributed to a single recurrent missense mutation: T790M within the EGFR kinase domain, which occurs in around half of resistant cases.18,19 Other mutations exist, including EGFR point mutations, EGFR amplification, bypass tracks and 15–20% unknown mechanisms.20 Several other agents are in clinical development.
Second-generation irreversible pan-HER EGFR TKIs, such as afatinib,21 dacomitinib22 and neratinib,23 do not seem to be highly effective after progression on first-generation TKI. As a result, third-generation EFGR inhibitors have been developed that selectively target EGFR-activating mutations (del19 and L858R), preserving their affinity in the presence of the T790M resistance mutation, but relatively sparing of EGFR wild type (WT) kinase. The most advanced of these are osimertinib (AZD9291)24 and rociletinib (CO-1686).25 In a phase I study, osimertinib showed a response rate of 61% and a progression-free survival (PFS) of 9.6 months among 127 T790M-positive patients previously treated with EGFR TKIs.26 The latest data from the two phase II studies (AURA extension and AURA2) showed a consistent efficacy and tolerability profile.27 Osimertinib received approval from the US Food and Drug Administration (FDA) in November 2015 and the European Medicines Agency (EMA) in December 2015 for EGFR T790M-positive NSCLC progressing after prior therapy with an EGFR TKI. Results of the confirmatory AURA3 phase III study are pending.
Rociletinib has also shown high efficacy (objective response rate [ORR] 59%, PFS 13.1 months) in T790M-mutated patients in a phase I/II study.28 However, due to a high proportion of unconfirmed responses, rociletinib has not received FDA approval yet and development has been halted. Interestingly, osimertinib and rociletinib also seem active in T790Mnegative NSCLC with lower response rates than in T790M-positive NSCLC. Unfortunately, resistance to third-generation EGFR TKIs occurs and new mechanisms of resistance have been found,29,30 which may differ for osimertinib, rociletinib and other agents in development. Of note, a few cases of NSCLC progressing after rociletinib have been shown to respond to osimertinib.31 Osimertinib and rociletinib are currently being tested in the first-line setting against first-generation TKIs. The results of these trials are eagerly awaited, as osimertinib have shown promising high efficacy (ORR 75%, 72% of PFS at 12 months) in preliminary data of first-line cohorts.32,33 The right sequence of EGFR TKIs is thus a big challenge. The choice may be guided by response to brain metastases: osimertinib and rociletinib appear to have almost as high efficacy in patients with brain metastases than without.34,35 Other agents in clinical development include HM6171336 and EGF816.37
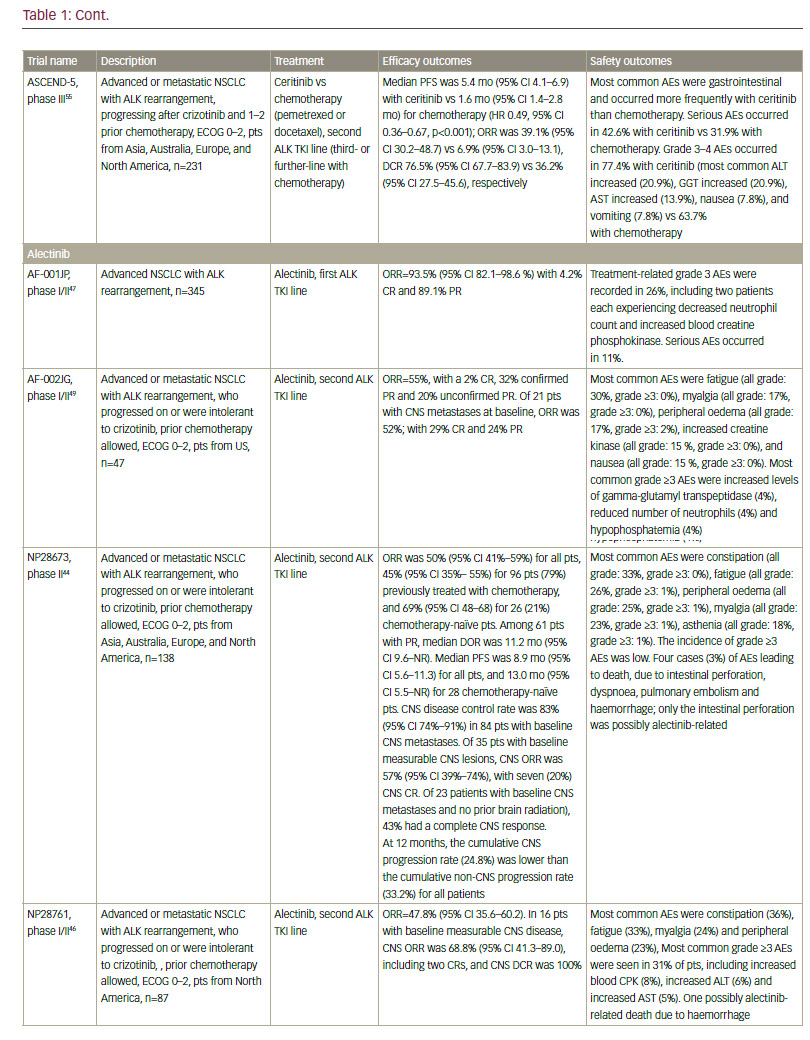
The ALK fusion gene is present in about 5% of NSCLC cases. The ALK inhibitor crizotinib received FDA approval in 2013, and has demonstrated ORR of over 70% in phase III studies in both first- and second-line settings.38,39 However, again, resistance inevitably develops after less than a year.20 Increased understanding of the mechanism of resistance has led to the development of second-generation ALK inhibitors. Ceritinib40–43 and alectinib44–47 are effective in more than 50% of patients who progressed on or were intolerant to crizotinib, and have received FDA approval in this second-line treatment setting.
In addition, ceritinib and alectinib have demonstrated remarkable activity in patients with brain metastases.40,48–55 By contrast, studies of crizotinib showed low brain response rates.56 In recent phase I/II clinical trials, the dual ALK/EGFR inhibitor brigatinib (AP26113), showed significant antitumour activity in ALK-positive NSCLC patients with brain metastasis following crizotinib.57 Subsequently, the large (n=222) phase II ALTA trial recruited advanced ALK+ NSCLC patients whose disease progressed on crizotinib and who had received no other ALK TKI. Recently presented data showed that brigatinib gave substantial responses.58 A phase III study is planned, and brigatinib and has received breakthrough therapy designation by the FDA.59 Other second-generation ALK inhibitors in clinical development include the dual ALK/ROS 1 inhibitor lorlatinib (PF-06463922), which has demonstrated durable clinical responses in a phase I/II study of ALK+ and ROS1+ NSCLC patients, most of whom had CNS metastases and had received at least one prior TKI;60 X-396;61 ASP3026;62 TSR-011;63 CEP28122/CEP-37440; and entreclinib.
Second-generation ALK inhibitors are also promising as first-line treatment options. The phase III J-ALEX study randomised patients without prior ALK inhibitor treatment to alectinib or crizotinib. Recently released data from this study shows that alectinib demonstrated significantly prolonged PFS compared with crizotinib and was well tolerated.64 This study was specific for Japanese patients only, and was selecting patients based on stringent ALK positivity criteria (ALK centralised testing, immunohistochemistry [IHC] and fluorescence in situ hybridisation [FISH] or reverse transcription polymerase chain reaction [RT-PCR]). Around half of the patients had received one prior line of chemotherapy. Of note, slightly more patients in the crizotinib group had brain metastases at baseline. Ceritinib40,65 and brigatinib66 are also further currently being investigated as frontline therapy in ALK-positive NSCLC. Current available data on ALK inhibitors are summarised in Table 1.
Although ALK inhibitors are generally well tolerated, they target multiple pathways, and have the potential for a wide range of treatment-emergent adverse events (AEs), including gastrointestinal AEs and hepatotoxicity.67 In addition, individual drugs present with very different toxicity profiles, such as the report of interstitial lung disease (ILD) on brigatinib; increase
in creatine phosphokinase (CPK) and muscular AEs on alectinib; QT increase on crizotinib and ceritinib, and more gastrointestinal AEs on ceritinib than on other ALK inhibitors. However, the majority of toxicities are reversible, manageable with proactive monitoring and treatment, and not severe if treated promptly.67
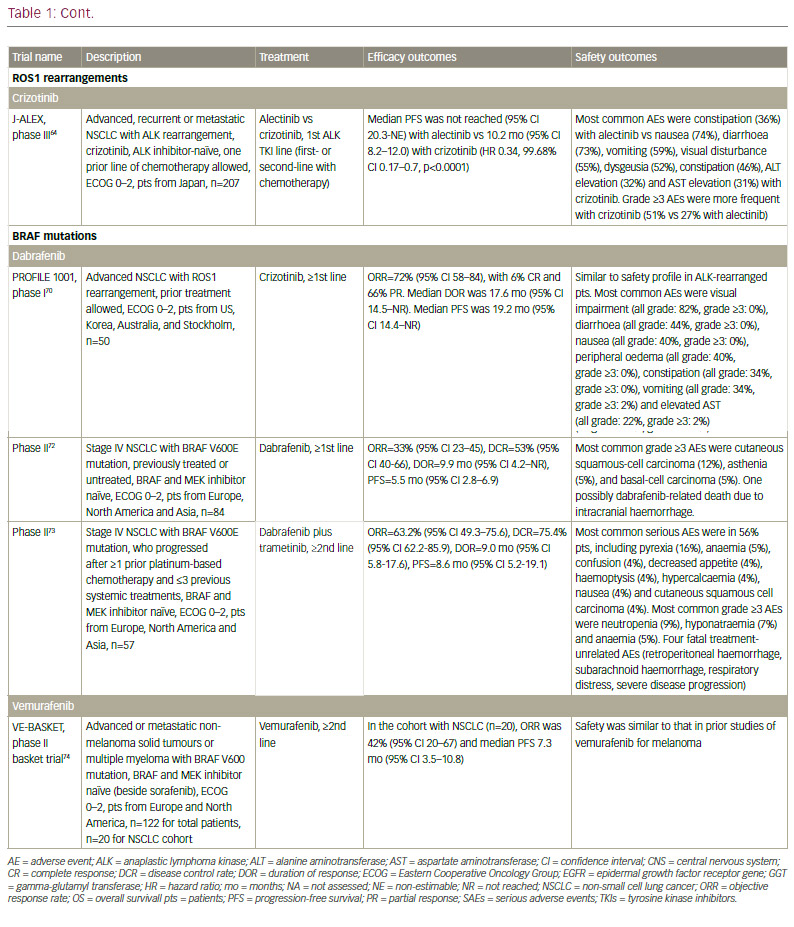
It must be noted that resistance to second-generation ALK inhibitors also occurs and novel ALK mutations have been detected.68 In addition, new mutations may confer sensitivity to other ALK inhibitors.69 Ideally, subsequent biopsies should be performed in order to determine the optimum sequencing of ALK inhibitors. However, very few centres perform repeat biopsies at relapse. Determining optimal sequencing of ALK inhibitors in the clinic is an important emerging question that warrants further study. Among other gene mutations in NSCLC, the most important include ROS1 fusions, for which crizotinib was approved after demonstrating an ORR of 72% in patients with advanced ROS1-rearranged NSCLC.70 Mutations of the BRAF gene have been identified in up to 3% of patients with NSCLC.71 Monotherapy with dabrafenib demonstrated a disease control rate of 53% at 12 weeks and a median duration of response of 9.9 months in previously treated patients.72 This agent has been shown to be even more effective when combined with trametinib.73 In addition, in a phase II study, 42% of a cohort with NSCLC showed a response to vemurafenib.74 Resistance mechanisms to this MAPK pathway multilevel inhibition are under investigation.
Other oncogenic alterations are currently being investigated; these include RET fusions, neurotrophic tyrosine kinase receptor type 1 (NTFK1) fusions, MET mutations or amplification, fibroblast growth factor receptor 1 (FGFR1) amplification, as well as human EGFR 2 (HER2) mutations.75
In summary, targeted therapies offer the opportunity to target specific genetic driver alterations, but they have limitations. Known mutations are not present in many cases: genetic changes have been identified in patients with non-squamous NSCLC, while EGFR and ALK mutations are rarely seen in squamous NSCLC.76 To date, no targeted interventions are available for patients with squamous NSCLC. In addition, a relatively small proportion of Caucasian patients with non-squamous NSCLC harbour EGFR mutations. Although 65% of patients present with a tumour characterised by an oncogenic alteration, many are still not treatable. More importantly, targeted therapies are not curative in patients with metastatic NSCLC and disease inevitably progresses after a median of 8–12 months, highlighting the urgent need for additional, more effective strategies. Current therapeutic approaches are therefore developing in another direction, utilising the immune response in solid tumours.
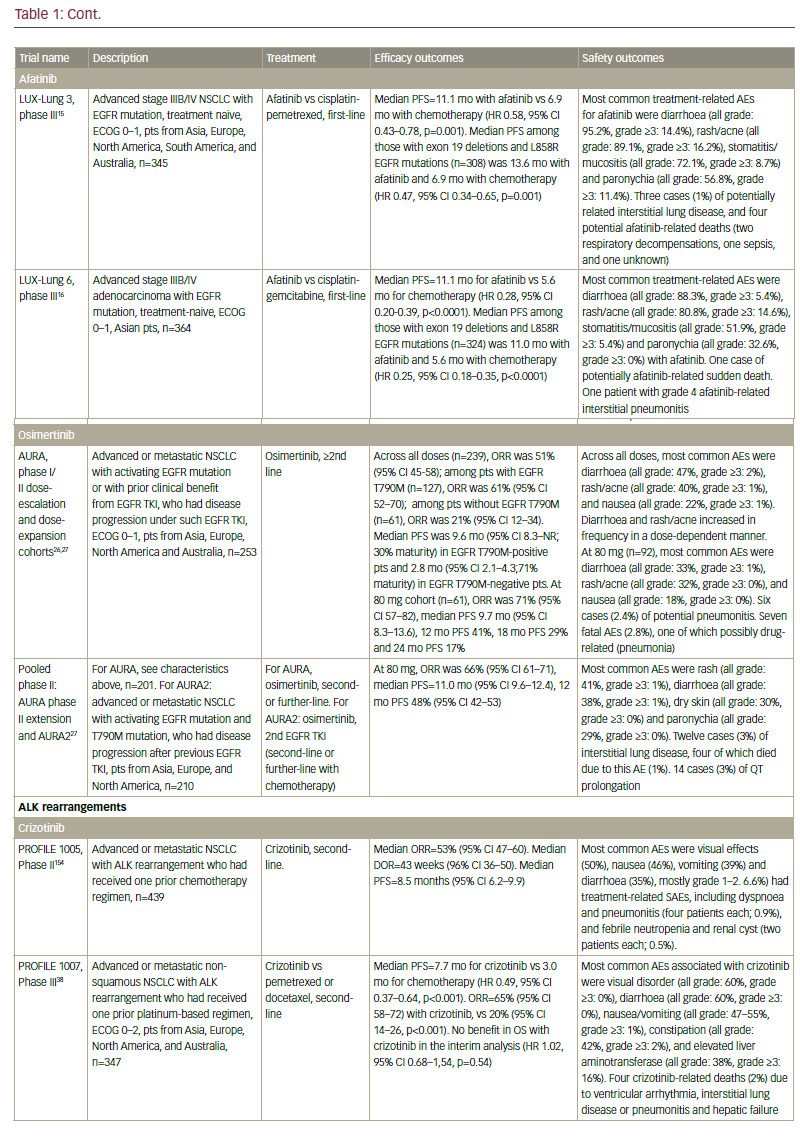
Immunotherapy in non-small cell lung cancer
Although NSCLC was not believed to be an immunogenic malignancy due to lack of efficacy of immunomodulatory cytokines such as interleukin 2 (IL-2) and interferon (IFN), and of early vaccines,77–79 recent data demonstrating the efficacy of immune checkpoint inhibitors have established the importance of the immune response in NSCLC (see Table 2). In addition, neoantigen-reactive tumour-infiltrating T cell lymphocytes (TILs), which can potentially induce tumour regression, were identified in NSCLC.80,81
Inhibitory immune checkpoints are inhibitory signalling pathways that down-modulate the immune system responses of T cells, blocking the inappropriate recognition of normal tissue by activated immune cells and preventing autoimmunity.82 Various checkpoint molecules have been identified, including cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1), T-cell immunoglobulin domain and mucin domain-3 (TIM-3), and lymphocyte-activation gene-3 (LAG-3), among others.82
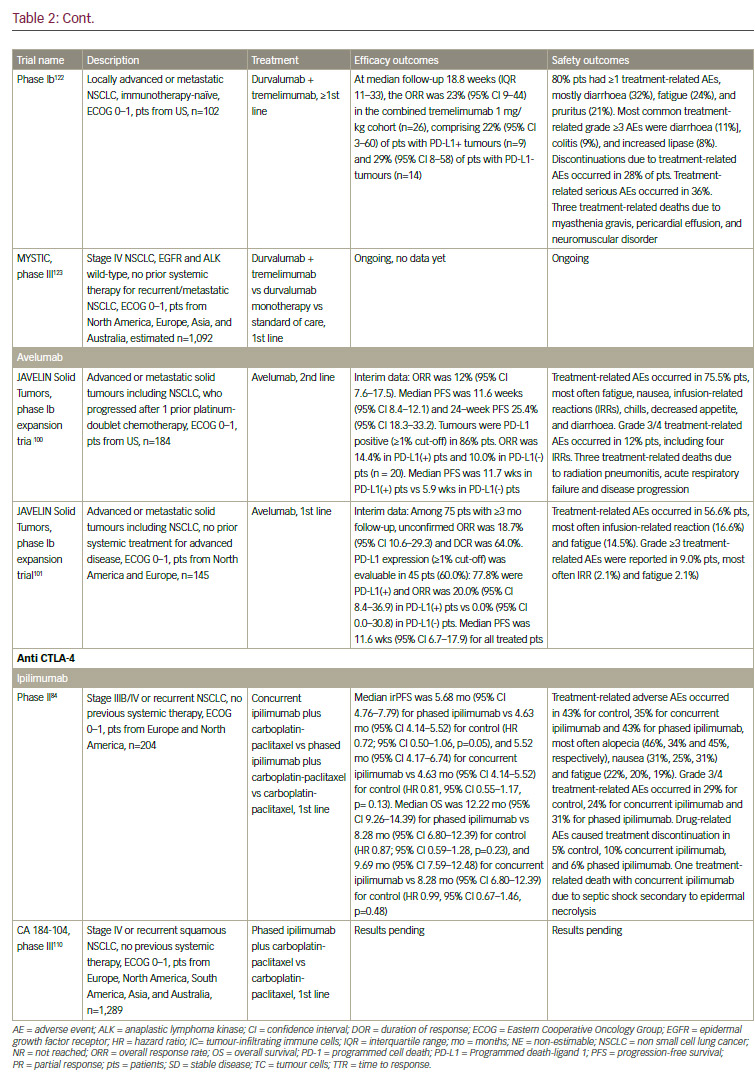
The use of CTLA-4 inhibitors is widespread in immunotherapy, and while the efficacy of ipilimumab (Yervoy®, Bristol‑Myers Squibb, New York, US) in melanoma is well established,83 its potential in NSCLC has not yet been fully explored. In a phase II trial, ipilimumab, in combination with first-line chemotherapy, improved PFS in patients with metastatic NSCLC.84 In a phase II study, tremelimumab did not demonstrate superiority over best supportive care in NSCLC patients but a partial response rate was seen in 4.8% versus 0% with best supportive care, suggesting that combined therapeutic approaches with tremelimumab warrant further investigation.85
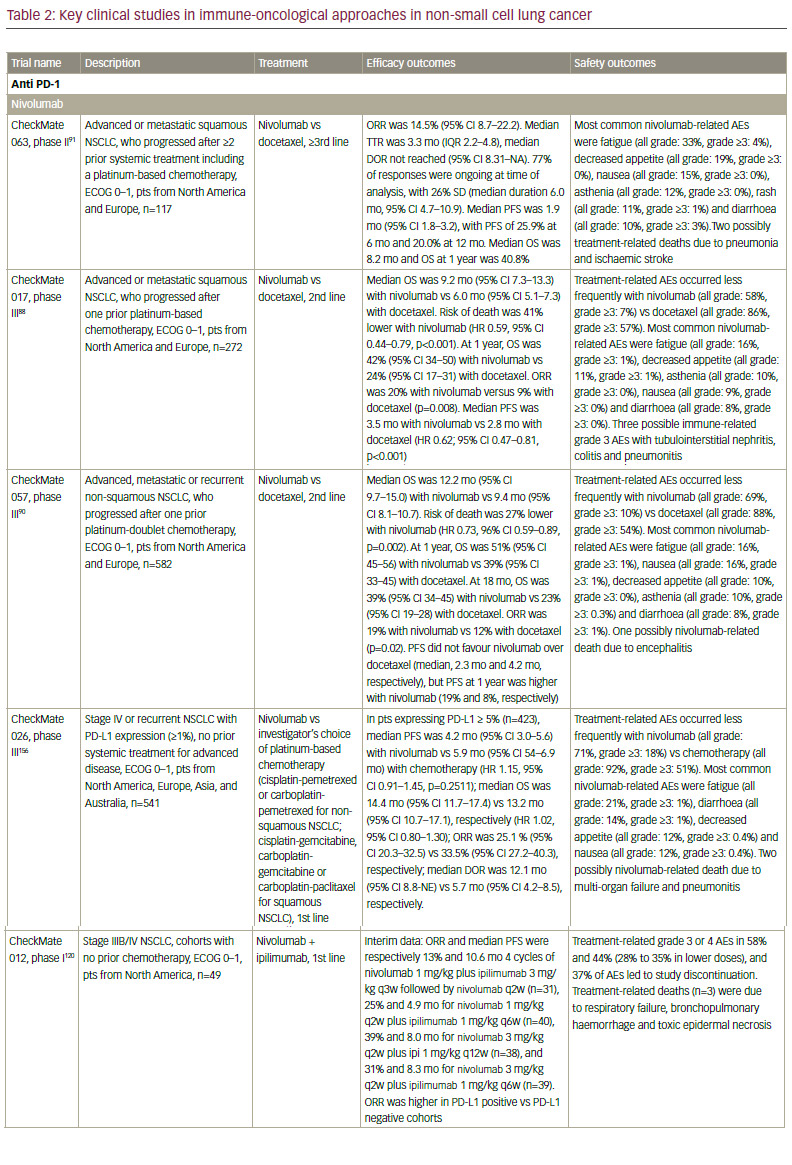
Many cells, including T cells, B cells, natural killer (NK) cells, NKT cells, dendritic cells (DCs), and macrophages, express the transmembrane protein PD-1. Cancer cells can affect the binding of the PD-1 receptor to one of two ligands, programmed death-ligand 1 (PD-L1) and PD-L2, on activated T cells, rendering the cell unable to exert its immunologic actions and thus enabling tumour cells to evade immunological surveillance.86,87 Therapeutic PD-1 blockade using the anti-PD 1 monoclonal antibody (mAb) nivolumab (Opdivo®, Bristol‑Myers Squibb, New York, US) has demonstrated efficacy in patients with refractory NSCLC. In March 2015, nivolumab became the first immunotherapy to be approved for NSCLC, when it received FDA approval for squamous cell NSCLC; in October, this approval was expanded to include non-squamous NSCLC. Nivolumab has also received approval from the EMA for squamous and non-squamous NSCLC, regardless of PD-L1 expression.
Regulatory approval was based on data from the phase III CheckMate 017 trial in previously treated patients with squamous cell NSCLC, showing that nivolumab significantly improved overall survival compared with docetaxel, regardless of PD-L1 expression level.88,89 The Checkmate 057 study found that nivolumab also improved survival over docetaxel in the second-line treatment of non-squamous cell NSCLC, with striking survival and response benefit in patients with PD-L1 expression greater than 1%, but equivalent overall survival to docetaxel in the patients who were PD-L1 negative.89,90
A phase II study of nivolumab in refractory patients with squamous cell NSCLC, CheckMate 063, included patients who had received two or more previous treatments; two-thirds of patients had progressed following three systemic regimens. Although the response rate was only 14.5%, almost all responders had ongoing responses (median duration of response not reached).91 Nivolumab was associated with two treatment-associated deaths caused by pneumonia and ischaemic stroke that occurred in patients with multiple comorbidities in the setting of progressive disease.91 However, other AEs associated with nivolumab are manageable.92
Data are also emerging for other anti–PD-1 agents. In October 2015, pembrolizumab (Keytruda®, Merck & Co., Inc., New Jersey, US) received accelerated FDA approval for the treatment of NSCLC after failure of first-line therapy that includes platinum-based chemotherapy or after anti-EGFR or anti-ALK therapy in oncogene-addicted NSCLC patients with appropriate mutations, and with 50% PDL-1 expression or more. In a phase I study (KEYNOTE-001) in patients with advanced NSCLC, pembrolizumab showed a response rate of 19.4%, a duration of response of 12.5 months, as well as a tolerable toxicity profile.93 Recent long-term data (median follow-up duration 23.1 months) showed that the overall survival was 22.1 months for treatment-naive patients and 10.6 months for previously treated patients. The survival benefit seems to raise with increasing PD-L1 positivity (that is, patients with PD-L1 ≥1%).94 In an open-label, phase II/III study KEYNOTE-010, patients with PD-L1 positive (PD-L1 ≥1%) NSCLC were randomised to pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel. Overall survival was significantly longer for pembrolizumab 2 mg/kg versus docetaxel and for pembrolizumab 10 mg/kg versus docetaxel (in patients with PD-L1 >1% only),95 leading to EMA registration this year.
It is worth noting that histology is not useful for selecting patients for immunotherapy: clinical trial data have indicated that these agents have similar efficacy for both squamous and non-squamous NSCLC.96,97
PD-L1 is one of two ligands that interact with PD-1 (another one is PD-L2) to render T cells ineffective, and is expressed on tumour cells and tumour-infiltrating immune cells. To date, no significant difference in activity or toxicity profile has been observed between anti-PD1 and anti- PD-L1 compounds. Differential activity attributable to the mechanism of action might be observed in the combination setting in the future
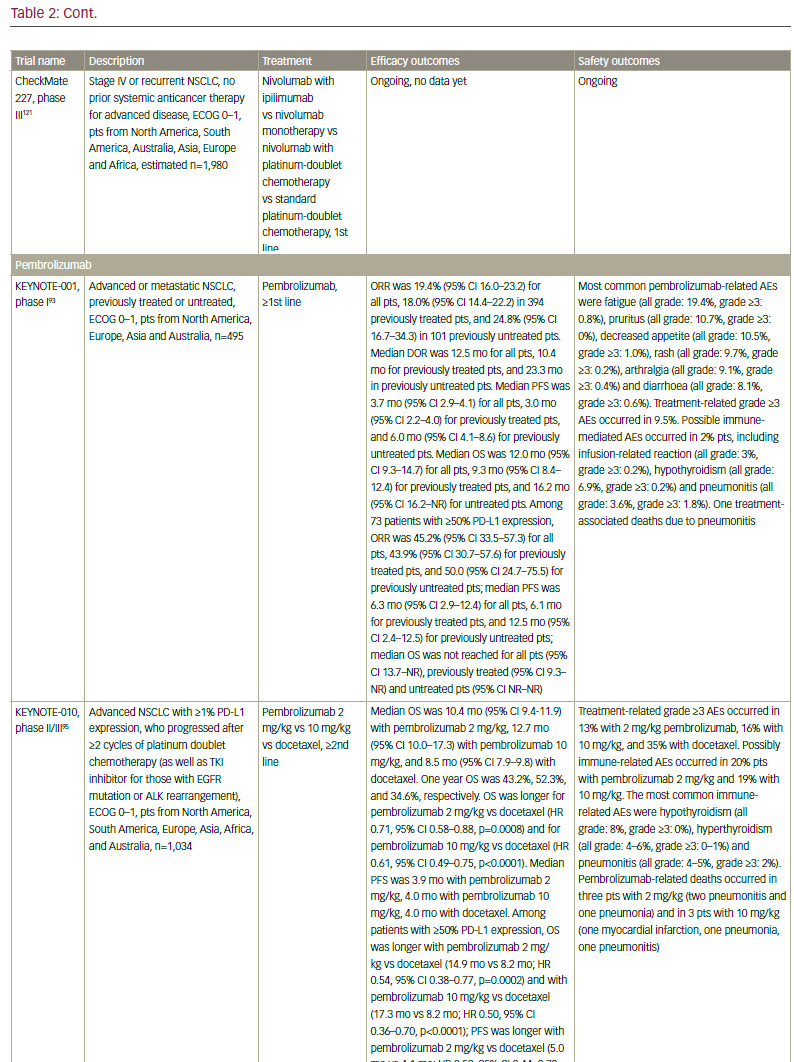
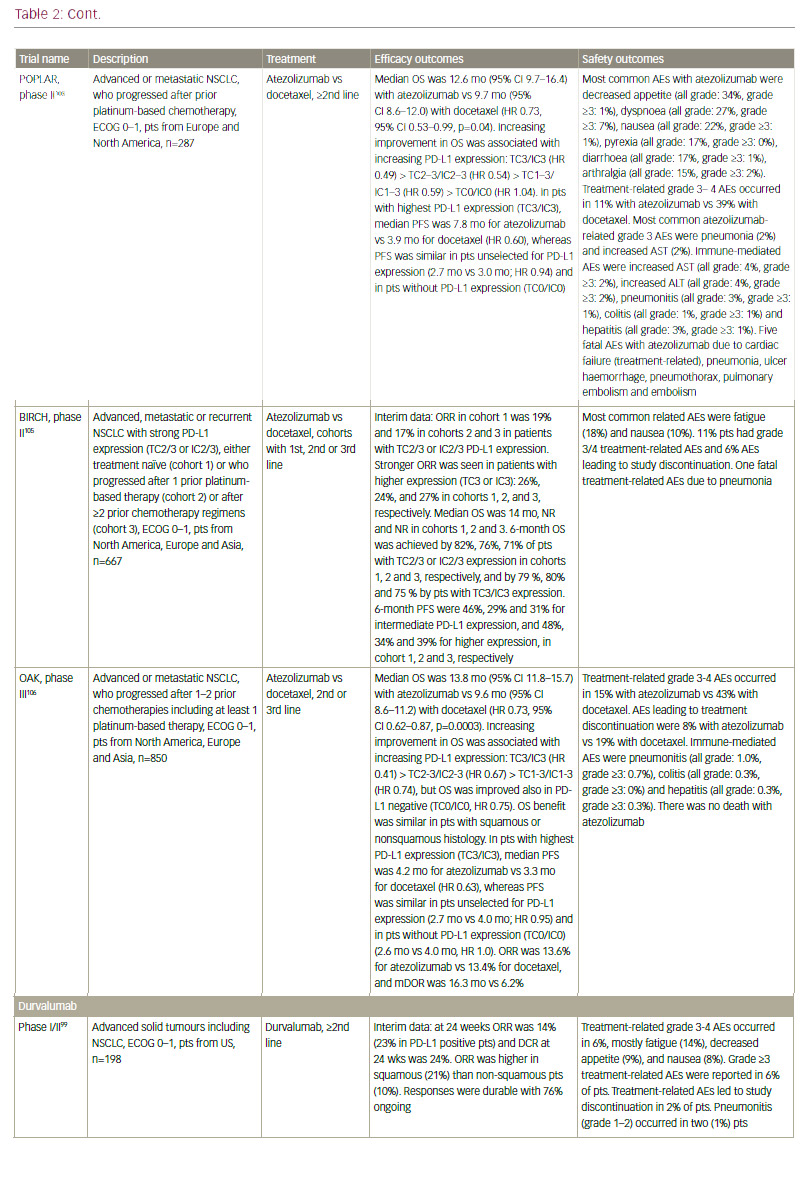
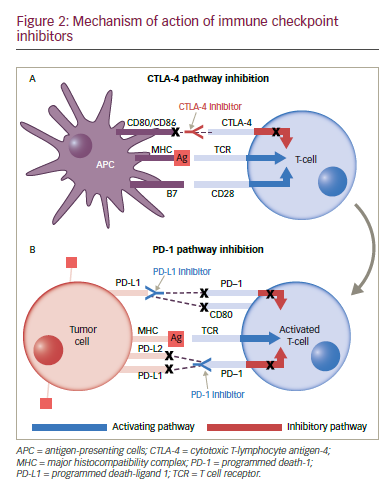
though. Anti-PD-L1 agents are also in active clinical development. These include atezolizumab (MPDL3280A),98 durvalumab (MEDI4736),99 and avelumab (MSB0010718C).100,101 Atezolizumab (MPDL3280A) has shown clinical efficacy in both chemotherapy-naïve and previously treated NSCLC.102 In the phase II POPLAR trial, atezolizumab significantly improved overall survival and overall response rates versus docetaxel in patients with non-squamous and squamous NSCLC with strong PD-L1 expression.103 Extended follow-up revealed further separation later in the OS curves and increased benefit with atezolizumab versus docetaxel.104 In the phase II BIRCH trial, atezolizumab also met its primary endpoint in patients with strong PD-L1 expression.105 In the recently presented phase III OAK trial, atezolizumab showed an improved overall survival versus docetaxel in patients with advanced NSCLC who progressed after one or two prior chemotherapy. Interestingly, the OS benefit was seen regardless of PD-L1 expression, histology, sex or smoking status.106 In October 2016, atezolizumab received FDA approval for the treatment of patients with metastatic NSCLC whose disease progressed during or following platinum-containing chemotherapy.
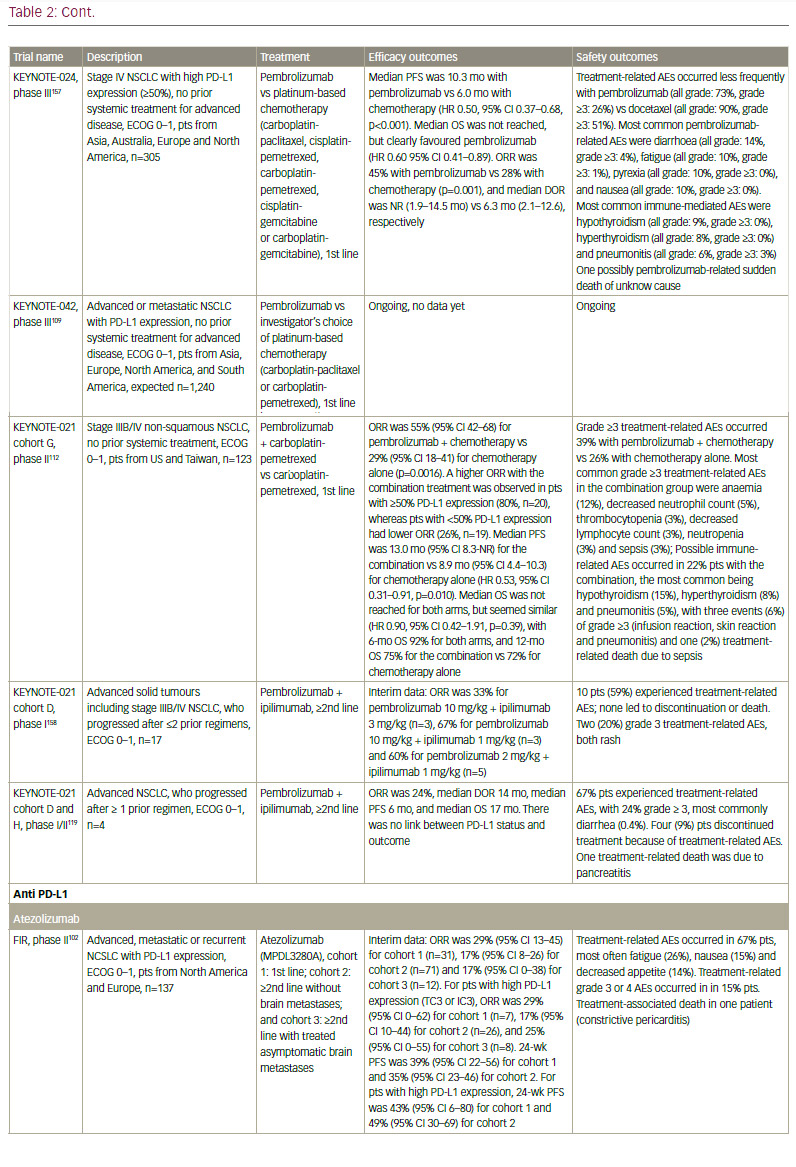
In the first-line setting, two phase III clinical trials testing anti-PD-1 monotherapy have been recently presented at the European Society for Medical Oncology (ESMO) 2016: CheckMate 026 and KEYNOTE-024. CheckMate 026 investigated the efficacy of nivolumab compared to platinum-based chemotherapy in untreated advanced NSCLC with PD-L1 expression (defined as present in ≥5% tumour cells). The trial did not meet its primary endpoint, which was defined as PFS assessed by an independent radiology review committee in patients with ≥5% PD-L1 expression, while all patients with ≥1% PD-L1 could be enrolled: the median PFS was 4.2 months with nivolumab compared with 5.9 months with chemotherapy (hazard ratio [HR] 1.15).107 The median OS was similar in both arms (14.4 months versus 13.2 months, HR 1.02), possibly reflecting the high rate of crossover to immunotherapy on the chemotherapy arm (60.4%). KEYNOTE-024 investigated the efficacy of pembrolizumab compared to platinum-based chemotherapy in untreated advanced NSCLC with high PD-L1 expression (defined as present in ≥ 50% of tumour cells, which represented about 30% of patients).108 The trial met its primary endpoint, showing an improved PFS with pembrolizumab compared to chemotherapy (10.3 months versus 6.0 months, HR 0.50). A significant benefit in response rates, duration of response and overall survival (not reached in both arms, HR 0.60) was observed, even though the crossover rate was high (44% from chemotherapy to pembrolizumab). This is a landmark result in highly selected PD-L1 positive patients that will change clinical practice in the first-line setting. More studies are required to confirm these findings in patients with high PD-L1 expression, as well as in those with lower PD-L1 expression. The result of the phase III KEYNOTE-042 (first-line pembrolizumab versus platinum-based chemotherapy)109 are awaited. Moreover, more research is needed to assess the difference with CheckMate 026 results and whether more stringent selection of patients may also confer a benefit of nivolumab compared to chemotherapy.
Combined immunotherapeutic approaches
Combined therapeutic strategies involving immunotherapy is an area of considerable interest. At present, there is no approved biomarker for response to anti-PD-1/anti-PD-L1 agents. The majority of PD-L1 positive patients do not respond to checkpoint inhibitors. Furthermore, more than half of patients with NSCLC have tumours that are PD-L1 negative, some of which have responded to checkpoint inhibitors. Therefore, PD-L1 expression is not the only factor determining response. Immunotherapy combinations may be employed in PD-L1 negative or undetermined tumours and may involve chemotherapy and other approaches, for example, antiangiogenic compounds or radiotherapy. Tumour-specific antigen released during chemotherapy-induced tumour necrosis may increase tumour-specific immunity and therefore enhance the efficacy of ipilimumab or other immunotherapeutics.
Several clinical studies are investigating combined approaches. As discussed earlier, ipilimumab has shown efficacy in treating patients with metastatic NSCLC in combination with first-line chemotherapy84 and is currently being investigated in squamous NSCLC in a phase III trial.110 A phase III study is investigating the combination of atezolizumab and chemotherapy in chemotherapy-naïve patients with advanced NSCLC.111 The phase II KEYNOTE-021 cohort G study was recently presented at ESMO 2016, assessing the efficacy of adding pembrolizumab to carboplatin-pemetrexed compared to carboplatinpemetrexed in first-line untreated advanced non-squamous NSCLC. The pembrolizumab combination showed a significant increase in ORR (55% versus 29%, p=0.0016) compared to chemotherapy alone. A higher response rate (80%) was observed in a few patients with ≥50% PD-L1 expression treated with the combination. The pembrolizumab combination also improved PFS (13.0 months versus 8.9 months, HR 0.53) compared to chemotherapy alone, while OS seemed similar (HR 0.90). Grade ≥3 treatment-AEs occurred more frequently with the combination, but this did not seem to impact on treatment discontinuation nor treatment-related deaths.112 Results of phase III trials are awaited to confirm the efficacy of adding pembrolizumab to chemotherapy in the first-line setting, such as the ongoing phase III KEYNOTE-189 trial (platinum-pemetrexed chemotherapy plus or minus pembrolizumab in first line advanced non-squamous NSCLC)113 and KEYNOTE-407 (carboplatin with paclitaxel or nab-paclitaxel plus or minus pembrolizumab in first line metastatic squamous NSCLC).114
Another promising combination is that of immunotherapy and radiotherapy: ionising radiation generates inflammatory signals that may enable activation of tumour-specific T cells.115 A case study has found that radiotherapy can elicit an immune-mediated abscopal (that is, away from the target) effect in NSCLC, when combined with ipilimumab.116 Preclinical data have suggested that targeting both PD-L1 and CTLA- 4 may have additive or synergistic effects,117 leading to a number of clinical studies. The phase I/II KEYNOTE-021 study is evaluating pembrolizumab in combination with standard therapies (including cohorts with pembrozilumab plus ipilimumab) compared to standard chemotherapy. In the phase I KEYNOTE-021 cohort D, this combination regime was feasible and has demonstrated clinical efficacy regardless of pembrozilumab dose or PD-L1 status in treatment-naive advanced NSCLC.118 However, in the follow-up phase I/II KEYNOTE-021 cohort D plus H, this combination has shown a similar response rate (24%) than pembrolizumab alone, with more treatment-related grade ≥3 AEs (24%).119 The CheckMate 012 trial is investigating the combination of ipilimumab and nivolumab as first-line treatment in advanced NSCLC. In a recent presentation, response rates of 13% to 39% were reported, with median duration of response not reached yet (median follow-up 16.6 months).120 CheckMate 227 is investigating nivolumab with or without ipilimumab, compared with standard platinum-doublet chemotherapy with or without nivolumab depending on the PD-L1 status in the first-line treatment of advanced or metastatic NSCLC.121 In a phase I study, the combination of durvalumab and tremelimumab showed a manageable tolerability profile, with antitumour activity in patients with locally advanced or metastatic NSCLC, irrespective of PD-L1 status.122 This combination is now being investigated in a phase III study, MYSTIC, comparing to durvalumab monotherapy or standard chemotherapy in first line advanced NSCLC.123
Lymphocyte-activation gene 3 (LAG3; CD223) is a co-inhibitory receptor expressed in activated T cells, Tregs, DCs and NK cells.52 Further work is needed to characterize LAG3 in NSCLC; an ongoing phase I study is investigating the role of BMS-986016, a LAG3 mAb with or without nivolumab in advanced solid tumours.124 OX40 is a co-stimulatory receptor that is transiently expressed by T cells upon antigen recognition. A phase Ib study is investigating the combination of atezolizumab and MOXR0916, a mAb that targets OX40, in patients with advanced solid tumours, including NSCLC. Preliminary data show that the combination is well tolerated.125
Rationale for selecting targeted and immunotherapies in non-small cell lung cancer
Targeted and immune-oncological approaches should not be regarded as competitive options for NSCLC; both should be considered as an option for treatment in any patient based on the patient’s disease characteristics. Targeted therapies are associated with higher response rates than immunotherapy – response rates typically exceed 50%, while up to 25% is more typical for immunotherapy. This reflects the different principles and mechanisms of actions of the two approaches. As of yet, immunotherapy should not be considered as first-line therapy in patients with oncogene driver/target mutations. The labels for nivolumab and pembrolizumab state that EGFR and ALK patients should be treated with targeted drugs first and fail before immunotherapy is considered.126,127
Since immune checkpoint inhibitors are highly active in a select group of patients, there is a need for predictive biomarkers. Certain gene mutations, including EGFR or ALK, are more prevalent in non-smokers, whereas the majority of patients with NSCLC are smokers.128 Anti-PD-1/anti- PD-L1 agents appear to be most effective in smokers, in whom somatic gene mutations are more abundant.129 Patients with no smoking history might present with a lower response rate to PD-1 pathway blockade, despite the facts that only a few patients have been reported to date, and that some long-lasting benefit has also been observed in some of these patients.130 It has thus been hypothesised that immunotherapeutic approaches are less effective in never-smokers with NSCLC as well as in patients with EGFR and ALK aberrations. Smoking history, as an indirect reflection of the patient mutation load, may therefore be useful in clinical decision-making.
In general, NSCLC is associated with a high mutation burden, especially in smokers,131–135 but there is a large variability within both tumour types and patients. Interestingly, the high mutation burden of NSCLC may correlate with higher neoantigen quantities,80,136 and with improved outcome under anti-PD-1 therapy.80 Moreover, neoantigen-specific T-cell reactivity was correlated with tumour regression after anti-PD-1 therapy, suggesting that anti-PD-1 therapy might enhance neoantigen-reactive T cells in NSCLC.80
Expression of PD-L1 has been associated with a higher response rate and overall survival in anti-PD-1 and anti-PD-L1 mAb treatment in some cases of non-squamous NSCLC.90,98,137–139 However, the use of PD-L1 as biomarker is limited by the fact that PD-L1-negative tumours have responded to anti-PD-L1 treatment, and heterogeneity in tumour expression of PD-L1 exists.140,141 Further studies are required to determine the value of this marker in prediction of response to treatments targeting this pathway. Measurement of TILs subpopulations is also a potentially useful strategy under investigation for predicting response to checkpoint inhibitors.142
There is a lack of clear knowledge of the activity of immunotherapy in oncogene addiction, that is, dependence of cancer cells on a single oncogenic protein for sustaining growth and proliferation. A recent study found that median PD-1 expression was highest in males, in current smokers, in individuals with adenocarcinoma histology, in EGFR wild type ALK negative patients, and in patients harbouring KRAS mutations. By contrast, PD-L1 expression was highest in females, in never/former smokers, in adenocarcinoma histology, in patients harbouring EGFR mutations, and in patients with ALK translocations.143
Combined targeted and immune-oncological approaches
Targeted therapy and immunotherapy may have complementary roles in cancer treatment. It has been postulated that tumour cell death resulting from targeted therapies causes antigen release. These antigens are taken up by antigen-presenting cells that activate T cells, leading to the upregulation of CTLA-4 and PD-1. Immune checkpoint therapy prevents attenuation of T cell responses, allowing T cells to kill tumour cells.144 Combined targeted and immune-oncologic approaches therefore offer the potential to extend the duration of treatment response and delay development of resistance. The association between PD-L1 and the presence of EGFR mutations suggests that combination of PD-1 blockade and EGFR TKIs may be a promising therapeutic strategy.143,145
It is essential to evaluate doses and dosing schedules in the evaluation of combined therapeutic regimes. Although toxicities may be nonoverlapping, it is impossible to predict whether activity and risks might be attenuated in combined approaches. In two clinical trials of BRAF inhibitors in melanoma, liver toxicity due to the combination of vemurafenib and ipilimumab led to discontinuation of the trial,146 while the combination of dabrafenib and ipilimumab appears to be well-tolerated.147 Recently, two clinical trials of the combination of osimertinib and durvalumab were halted following reports of ILD.148 A recent analysis of these data found that the combined ILD rate of 38% with five cases of grades 3/4 reported for the combination was much greater than either sole agent.149 However, there was no apparent increase in the severity of ILD, and tumour response rate suggests encouraging clinical activity of osimertinib plus durvalumab in EGFRmutant NSCLC. According to the authors, the tolerability and safety of combining these agents warrants further investigation.149
The combination of immunotherapy and targeted therapies is also potentially useful for patients with oncogenic alterations who are refractory to targeted treatment and do not present a treatable oncogenic resistance mediating alteration (such as T790M for EGFR TKI). However, it is important to establish the correct sequence of treatments; it is not known whether combined regimens should be employed as first-line therapy or in treatment-refractory patients.
Future perspectives
Among future strategies, the combination of vaccines and checkpoint inhibitors may be promising: cancer vaccine-based immunotherapy may overcome the resistance of certain cancers to immune checkpoint inhibitors, while immune checkpoint inhibitors may enhance the efficacy of the cancer-vaccine therapies. As an example, a recently initiated phase Ib/II study will investigate vaccination with viagenpumatucel-L (HS-110) in combination with multiple treatment regimens including nivolumab.150 In addition, molecular approaches to NSCLC are an area of active clinical development. These include targeting cancer stem cells,151 adoptive cell therapy with gene-modification of peripheral T-cell with engineered T cell receptors or chimeric antigen receptors.152,153
Summary and concluding remarks The one-size-fits all approach to cancer therapeutics is no longer applicable to NSCLC. We are moving into an era of personalised treatment of NSCLC. In addition to histological subtyping, NSCLC should now be further sub-classified by driver mutation if present. Optimal management of NSCLC in the future will require screening for a range of biomarkers that help to predict sensitivity to targeted therapy; point mutations and rearrangements in specific genes including HER2, BRAF, NUT, MET, ROS1, DDR2, FGFR1 and KRAS, might potentially provide useful information for clinical decision making.154
The subset of patients with treatable oncogenic alterations should receive appropriate targeted therapies. Within this group, specific resistance mechanisms have been identified; however, these can be treated specifically. The majority of patients with NSCLC are still not defined by treatable oncogenic alterations. Such patients may be treated using chemotherapy but are ideal candidates for immune-oncologic treatment. The PD-1 inhibitors, nivolumab and pembrolizumab, have shown a survival benefit in NSCLC. However, not all patients respond to immunotherapy and at present, the reliability and availability of predictive biomarkers is poor. There is an urgent need to identify high precision biomarkers to select patients for immune-oncologic treatment. Expression of PD-L1 has been associated with higher response rate and overall survival in several clinical trials evaluating anti-PD-1 and anti-PD-L1 mAbs, but further studies are needed to establish its potential role as a predictive biomarker.
Finally, the combination of immunotherapy and targeted therapies is currently an area of active clinical research. These two distinct approaches – one that targets the cancer and the other that targets the patient – may ultimately merge to provide an individualised approach to NSCLC therapy