Neuroendocrine neoplasms (NENs) are a group of rare and heterogeneous malignancies that arise from neuroendocrine cells in most organs of the body. The presence, rather than absence of symptoms due to neuropeptides or hormones hypersecretion, distinguishes NENs in functioning and non-functioning tumours, respectively. The term NEN is used to denote any type of neuroendocrine malignancies, irrespective of histological grade and clinical behaviour.1 Gastroenteropancreatic (GEP) NENs are classified as neuroendocrine tumours (NETs) and neuroendocrine carcinomas (NECs).2 Thoracic NENs, the majority of which are bronchial, are classified as carcinoids and carcinomas (see Table 1).3. 5 The terms ‘tumours’ for GEP and ‘carcinoids’ for lung represent the low/intermediate grade of malignant NENs.
Bronchial carcinoids (BCs) represent less than 3 % (1–2 % typical carcinoids [TCs] and 0.1–0.2 % atypical carcinoids [ATCs]) of all lung cancers, but they comprise around 20–30 % of all lung NENs. Small cell lung carcinoma (SCLC) is the most common lung NEN (>80 %), and accounts for 15–20 % of lung cancers, while large cell neuroendocrine carcinoma (LCNEC) is rarer, comprising 1.6–3 % of lung cancers. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) is a very rare but well-recognised pre-invasive lesion for BCs.6 BCs can be associated with a hyper-secretion syndrome, although this is more commonly seen in GEP NENs. Carcinoid syndrome (fewer than 5 % of cases), related to serotonin, is the most common one, and it is usually atypical. Cushing syndrome is rarer, related to ectopic adrenocorticotropic hormone (ACTH) production.7
BCs have a peak of incidence in the seventh decade.8,9 Data from the Surveillance, Epidemiology, and End Results (SEER) programme in the US suggests there is a growing incidence of BCs, with a fivefold increase reported between 1973 and 2004.10 This statistic could also be linked to an increased awareness and to the development of improved diagnostic techniques. More recent data from a Canadian population-based retrospective cohort study (n=5,619) found that between 1994 and 2009, the incidence of NENs of all origin increased from 2.48 to 5.86 per 100,000 per year. However, the proportion of metastases at presentation decreased from 1994 to 2009 (from 29 % to 13 %). The overall incidence of metastases did not change (0.63–0.69 per 100,000 per year), suggesting that the reason for increased incidence is improved detection.11 In the same study, an increase in the incidence of BCs has been reported from 0.83 per 100,000 per year to 1.28 per 100,000 per year.11
The prognosis of BCs varies according to sex, age group, socioeconomic status and tumour stage.11 In the Canadian study, BCs at an earlier stage were associated with 59.7 % 5-year and 49.7 % 10-year survival.11 An Israeli multicentre study (n=142) reported 5-year survival rates for patients with TCs and ACs of 89 % and 75 %, respectively,12 while a European single-centre study (n=252,174 [69 %] with TC and 78 [31 %] with AC) of BCs reported a 5-year survival of 90 %.13 This variation in survival can be explained by different histological subtypes and disease stages in the various studies. TCs have a good prognosis since they have a low rate of recurrence after adequate resection, even if metastases in regional lymph nodes are present; a 5-year survival of 87–90 % has been reported.3,8 ACs are associated with a less-favourable prognosis and a 5-year survival of 44–78 %.3,8 However, distant metastases may occur many years after radical resection of the primary tumour. A 15- year follow-up is therefore recommended.3,7 Large-cell NEC has a poor prognosis with a 5-year survival of around 35 %,14 and LCNEC and SCLC have the worst prognosis.3,8,15 However, if we consider metastatic BCs, the median survival is 17 months and the associated 5-year survival rate is approximately 25 %.16
While no antitumour treatment options were validated for metastatic BCs, several therapeutic options exist, none of which is currently approved, except for somatostatin analogues (SSAs) in functioning BCs. In addition to SSAs, chemotherapy, interferon (IFN), molecular targeted agents (MTAs) and peptide receptor radionuclide therapy (PRRT) may also be considered. The European Neuroendocrine Tumor Society (ENETS) recently published a consensus document with recommendations for best practice in BCs.7 There is no evidence supporting a specific sequence or priority for any of these therapies. In addition, liverdirected treatments, including embolisations (TAE, trans-arterial embolisation), radiofrequency (TARF, thermoablation radiofrequency), and radioembolisation (TARE, trans-arterial radioembolisation) can be useful.17–20 Mammalian target of rapamycin inhibitors (mTOR-I) have been widely investigated in NETs and everolimus has been approved for the treatment of patients with progressive neuroendocrine tumours of pancreatic origin (PNET) that are unresectable, locally advanced or metastatic. This review aims to discuss the role of the mTOR pathway in the pathogenesis of BCs, as also preclinical and clinical evidence supporting the use of mTOR-Is in this disease.
The Role of mTOR Signal Pathway in Bronchial Carcinoids
The mTOR Signal Pathway
mTOR is an intracellular serine/threonine kinase acting as a key regulator in cell survival, proliferation and apoptosis. Its interaction with multiple partners leads to the formation of two multi-protein complexes named mTOR complex 1 (mTORC1) and 2 (mTORC2). mTORC1 consists of mTOR, the regulatory-associated protein of mTOR (RAPTOR) and the target of rapamycin complex subunit LST8. mTORC1 is activated by multiple extracellular growth factors via the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, and it plays a key role in modulating cellular proliferation through the interaction with its downstream targets, including the eukaryotic translation initiation factor 4E binding protein-1 (4EBP-1) and ribosomal S6 kinase-1 (S6K1).21 Conversely, the association of mTOR with target of rapamycin complex subunit LST8, rapamycin-insensitive companion of mTOR (RICTOR) and mitogen-activated protein kinase-associated protein-1 forms mTORC2. Although its role is still poorly understood, mTORC2 is known to directly phosphorylate protein kinase B (AKT) in the PI3K pathway. S6K1 from mTORC1 inhibits the PI3K/AKT pathway via suppression of insulin receptor substrate-1 (IRS-1); thus, the mTORC1/mTORC2 complex is both an upstream and downstream regulator of cellular function, and
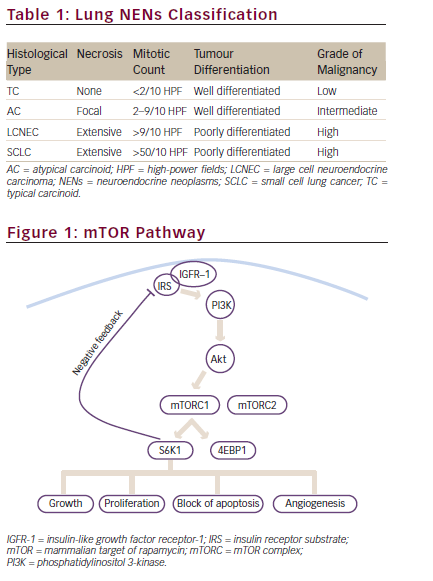
regulates cell survival, cytoskeletal remodelling and cell migration (see Figure 1).22,23 Binding of insulin or insulin-like growth factors (IGFs) to their receptors leads recruitment of PI3K to the cell membrane, activating AKT (protein kinase B). Phosphatase and tensin homologue (PTEN) is an inhibitory regulator of the PI3K/AKT/mTOR pathway that antagonises the action of PI3K.
Activation of mTOR contributes to the pathogenesis of many tumour types, and several findings have suggested that the mTOR pathway plays a key role in the development of NENs. Improved understanding of molecular genetics has shown that NENs are common in familial cancer syndromes arising from mutations in genes encoding proteins that lie upstream from mTOR.24 Moreover, a study performing exome sequencing reported somatic mutations in genes involved in the mTOR pathway in 14 % of sporadic pancreatic NETs.25
The mTOR Pathway in Bronchial Carcinoids
The proven efficacy of everolimus and the identification of multiple germline and somatic mutations in the PI3K/AKT/mTOR genes in pNETs highlight the relevance of this pathway in human endocrine tumours, leading to the exploration of its possible role in BCs. The first evidence of activated mTOR pathway in lung NENs came from a large Italian multicentric series of 218 surgically resected lung NENs patients, including both low- to intermediate-grade and high-grade tumours. The authors reported a differential activation of the mTOR pathway in the spectrum of lung NENs, with higher levels of phospho-mTOR (p-mTOR) and p-S6K expression by immunohistochemistry (p<0.001) in the lowto intermediate-grade tumours compared with the high-grade group
(p=0.001).26 Interestingly, the low p-mTOR expression in BCs was also reported to correlate with recurrent disease, and survival (p=0.005).27
Preclinical Studies of mTOR Inhibitors in Bronchial Carcinoids
Preclinical Evidence of Activity
The availability of data suggesting the involvement of mTOR signal pathway in BCs has led to studies investigating the activity of mTORIs in this setting. mTOR-Is, including rapamycin and its analogues, temsirolimus, everolimus and ridaforolimus, exert their activity in cancer by interacting with the FK506 binding protein and generating a complex that binds to mTORC1, inhibiting downstream signalling (see Figure 1).28
In 2010, Zatelli et al. observed an anti-proliferative effect of everolimus in human BC tumour cells associated with a reduction in chromogranin A level, VEGF secretion and cell viability, probably mediated by a mechanism involving IGF1 signalling.29 mTOR expression levels together with aggressive clinical features (i.e. AC, larger tumour size, higher number of mitoses) were found to be associated with an enhanced sensitivity to everolimus. Of note, previous reports showed that SSAs, such as pasireotide, were also associated with a decrease in VEGF expression and circulating IGF1 level, suggesting that a combined treatment approach might improve the outcome through an additive or synergistic effect.30 The same group confirmed everolimus activity on BC primary cultures and found a correlation between viability reduction and apoptosis activation.27
Molecular Bases for mTOR Inhibitor Resistance
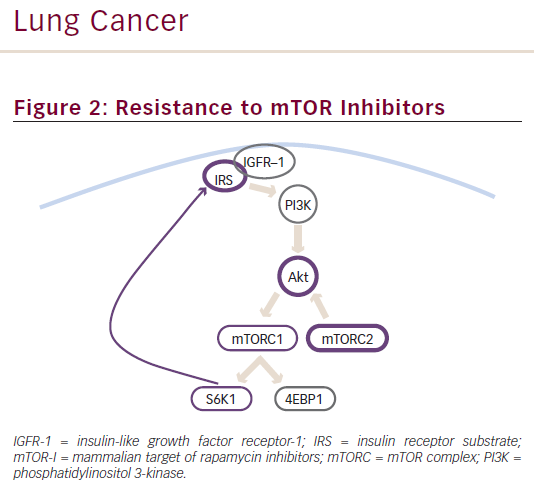
Attempts have been made to clarify the molecular bases of resistance to treatment with mTOR-Is and identify possible strategies to overcome this limitation. Although everolimus resistance is not yet completely understood, two potential mechanisms have been proposed: AKT activation by means of mTORC-2, and IGF1-IGF receptor (IGFR) pathway activation by means of phosphorylated S6K1 (see Figure 2).31,32 As previously stated, everolimus is selective for mTORC-1 alone and also inhibits the downstream molecule S6K1. The latter normally exerts a negative feedback on the PI3K/AKT/mTOR pathway activated by growth factors. Therefore, inhibiting S6K1 can reactivate mTOR, leading to everolimus resistance.33
A possible approach to overcome mTOR-Is resistance is the use of multitargeted inhibitors. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K and mTOR signalling activation and inhibits the growth of cancer cells with activating PI3K mutations.34 NVP-BEZ235 activity has been reported in BCs cell lines.33,27,35 A study in everolimus-resistant human BC tissues found that NVP-BEZ235 is twice as potent as everolimus in reducing cell viability and activating apoptosis in BC tissues sensitive to mTOR inhibitors, but no activity was reported in everolimus-resistant BC tissues and cell lines, suggesting that different pathways might be involved in resistance to mTOR-Is in BCs.27 Conversely, a preclinical study performed in PNET cells similarly showed that NVP-BEZ235 exerts a stronger anti-proliferative activity compared with everolimus, but reported that the combined administration of the two inhibitors exhibit a synergic effect, with activity also reported in everolimus-resistant cells.36
Clinical Evidence Supporting the Use of Everolimus in Bronchial Carcinoids
Clinical Evidence of Everolimus as a Single Agent in Bronchial Carcinoids
Pancreatic NETs represent the only NEN setting in which everolimus has received regulatory approval to date. Approval was based on the RADIANT-3 phase III trial (n=410), which compared everolimus versus placebo in patients with progressive, well/moderately differentiated, non-resectable locally advanced or metastatic pancreatic NETs. In this trial, the median progression-free survival (PFS) was 11.0 months with everolimus, compared with 4.6 months with placebo (hazard ratio [HR] for disease progression or death from any cause with everolimus, 0.35; 95 % CI 0.27 to 0.45; p<0.001), representing a 65 % reduction in the estimated risk of progression or death.37 The enrolment criteria included functioning and non-functioning tumours, and SSAs could be given in combination with everolimus based on the investigators’ decision.
BCs were included in an open-label, multicentre, phase II study, RAD001 in Advanced and Metastatic Silent Neuro-endocrine Tumors in Europe (RAMSETE), which evaluated the safety and efficacy of everolimus monotherapy (the use of SSAs was not permitted), in patients with advanced non-functioning, non-pancreatic NETs. Among the study population, 22/73 patients had a primary tumour in the lung, bronchus, thymus or mediastinum. In the whole population, a high rate of disease stabilisation (55 %) was achieved, with a PFS of 185 days. However, there was a high rate of discontinuation (75 %), with grades 3 and 4 adverse events (AEs) in 37 % of patients. In the lung subgroup a 63 % of stable disease and 189 days of median PFS were observed.38
The RADIANT-4 study was conducted to compare the antitumour activity of everolimus 10 mg/day plus best supportive care versus placebo plus best supportive care in patients with advanced non-functioning NETs of gastrointestinal or lung origin with progressive disease within the last 6 months.37,39 This study completed accrual recently, with a total of 302 patients, 90 (30 %) of whom had BCs. This is the largest BCs series ever included in a phase III trial. Recently presented data from this study, at the ECCO/ESMO 2015 meeting, showed that the median PFS was 11.0 months (95 % confidence interval [CI] 9.2–13.3) in the everolimus arm and 3.9 months (95 % CI 3.6–7.4) in the placebo arm (HR, 0.48; 95 % CI 0.35–0.67; p<0.001). HR in the lung subgroup was 0.50 (0.28–0.88). Everolimus was well tolerated and AEs were consistent with the known safety profile.40
Clinical Evidence of Everolimus in Association with SSAs in Bronchial Carcinoids
Octreotide has been reported to improve the antitumour effect of mTORIs in vitro and in vivo, probably by downregulating mTOR molecules upstream.41–43 A correlation between mTOR signalling pathway and
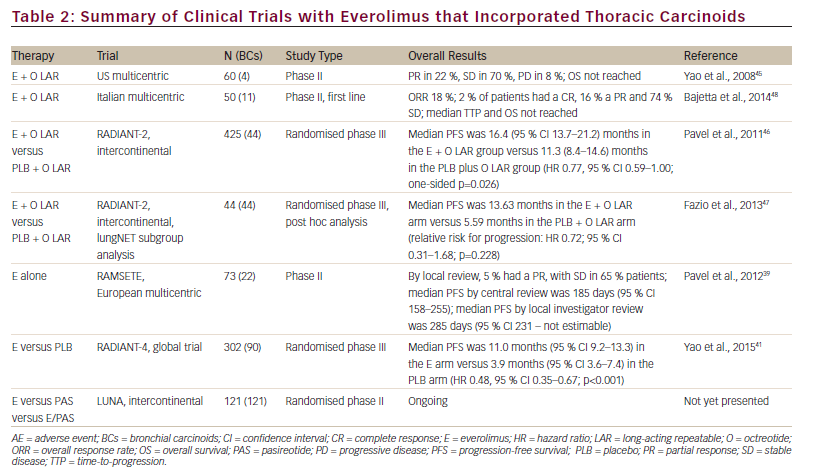
somatostatin receptors, subtype 2A and 3, has been observed in BCs.26 Clinical investigations of combinations of mTOR-Is and SSAs have been performed in NETs. In a single arm open-label phase II trial (n=60) the combination of everolimus and octreotide long-acting repeatable (LAR) showed promising antitumour activity (partial response in 22 %, stable disease in 70 % and progressive disease in 8 %) and was well tolerated; however, no conclusions can be drawn for BCs since only four patients with BCs had been included.44
The everolimus/octreotide LAR combination was studied in RADIANT-2, an international, randomised, double-blind phase III trial (n=429) comparing everolimus plus octreotide LAR versus placebo plus octreotide in NETs from different primary sites with carcinoid syndrome.45 Although according to a central radiology review, the combined therapeutic regimen resulted in an improvement in PFS (16.4 months, 95 % CI 13.7–21.2) in the everolimus plus octreotide LAR group and 11.3 (8.4–14.6) months in the placebo plus octreotide LAR group (HR 0.77, 95 % CI 0.59–1.00; p=0.026), this was not statistically significant considering the prespecified p value of 0.024. A subgroup analysis of the RADIANT-2 trial indicates that in patients with advanced lung NET (n=44), everolimus plus octreotide LAR improved median PFS by 2.4-fold; in particular, PFS was 13.63 months in the everolimus plus octreotide LAR arm (95 % CI 5.55–14.29) compared with 5.59 months in the placebo plus octreotide LAR arm (95 % CI 2.79–27.76) (see Table 2). Everolimus plus octreotide LAR was also associated with a 28 % reduction in the estimated risk for progression (HR 0.72, 95 % CI 0.31–1.68; p=0.228). Although not statistically significant this was a clinically meaningful improvement in PFS, better than the benefit seen in the overall RADIANT-2 population (a PFS increase of 5 months). The most frequently reported treatmentemergent AEs included stomatitis (69.7 % versus 0.0 %), rash (33.3 % versus 0.0%), diarrhoea (27.3 % versus 0.0 %) and asthenia (24.2 % versus 9.1%). These were similar to AEs reported in the overall RADIANT-2 population and consistent with the known safety profiles of everolimus and octreotide LAR.46 However, it should be noted that this is a small
retrospective analysis with imbalanced patient numbers between groups; the RADIANT-2 study did not stratify based on the primary tumour site, therefore there were more patients with BC in the everolimus plus octreotide LAR arm (33 patients) than in the placebo plus octreotide arm (11 patients).46
Recently, a phase II study (n=50, 11 had BCs) found that the everolimus/ octreotide LAR combination was active and well tolerated in treatmentnaïve patients with advanced NETs from different sites. The overall response rate () was 18 %; 2 % of patients had a complete response (CR), 16 % a partial response (PR) and 74 % stable disease (SD). At a median follow-up of 277 days, median time to progression and overall survival were not reached.47
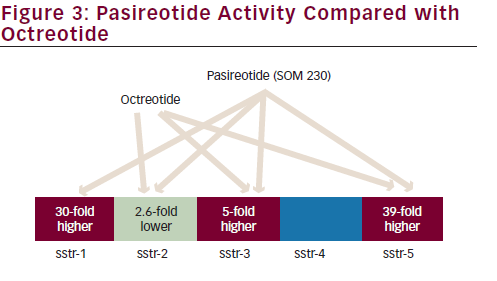
The everolimus/SSA combination is currently being investigated in lung NET within the multicentric international LUNA trial; this is a prospective phase II three-arm study comparing pasireotide LAR, everolimus alone and the combination of pasireotide LAR and everolimus, and it is the first study focused on BCs selectively. Pasireotide is a novel SSA that binds to a broader range (sstr-1,2,3,5) of somatostatin receptor subtypes than octreotide (see Figure 3).48 A phase I study has established the feasibility of combining pasireotide LAR and everolimus.49 The recruitment of the LUNA trial has been concluded sooner than expected with the inclusion of 120 patients with advanced metastatic BCs underlying the unmet need of therapeutic options in this disease. Results are awaited (see Table 2).50
In addition, the combined inhibition of mTOR and epidermal growth factor receptor (EGFR) has been shown to give synergistic effects in several cancer therapies, and a preclinical study suggested that this approach could be promising in the treatment of BCs.51 This is currently being investigated in a phase II clinical trial.52
Summary and Concluding Remarks
BCs represent a substantial proportion of NENs and given the lack of standard antitumour therapies there is a concrete need for effective therapeutic options. A considerable body of preclinical and clinical data suggests that mTOR-Is, specifically everolimus, are safe and efficacious in the treatment of BCs. A sub-analysis of the RADIANT-2 study suggested that the combination of everolimus and octreotide LAR could be beneficial in patients with advanced low- to intermediate-grade (unresectable or metastatic) functioning BCs. Although these results were not statistically significant and the study arms had an unbalanced distribution due to the lack of stratification for the lung primary site, this is an important finding. Following the recently presented data from the RADIANT-4 trial, including 30 % of patients with BCs in the study population, everolimus appears to be a promising therapeutic option for non-functioning advanced BCs. An ongoing clinical trial focused on BCs (the LUNA trial) will hopefully provide more information about everolimus as single agent and its combination with pasireotide in this rare condition.






