Prostate cancer is the most common cancer and the third leading cause of cancer-related death amongst men in Europe.1 Established treatment options for early-stage disease include surgery, radiation therapy and active surveillance,2 whereas patients presenting with advanced disease usually receive hormonal therapy using medical or surgical castration as the initial treatment.2,3 Unfortunately, most advanced prostate cancers acquire resistance to the initial hormonal therapy over 2–3 years, and progress to metastatic castration-resistant prostate cancer (mCRPC).2
Historically, the treatment of mCRPC has focussed on palliation, with available treatments providing only a modest clinical benefit.4–6 The taxane agent docetaxel remained the only modality that improved overall survival (OS) in patients with mCRPC for several years following its approval.7–9 However, in recent years, novel hormonal therapies targeting the androgen-receptor (AR) axis have become available for mCRPC, such as the CYP17 inhibitor, abiraterone acetate, and the anti-androgen, enzalutamide.6,10 There is a trend for these novel hormonal therapies to be used as an alternative to initial chemotherapy due to improved tolerability (e.g. compared with docetaxel),6,8 though the optimal sequence of AR-axis-targeting agents and taxane chemotherapy remains unclear.8 Retrospective studies have also indicated a decreased efficacy in second-line AR-axis-targeting therapy after progression on first-line therapy,8,11 suggesting shared mechanisms of resistance.10 It is therefore important to identify the optimal sequencing of AR-axis therapies in order to achieve the best clinical outcomes for patients with mCRPC. In this article, we review recent clinical evidence on the treatment sequencing of abiraterone acetate plus prednisone and enzalutamide and discuss how this might potentially impact the treatment paradigm for patients with mCRPC.
Treatment sequencing – retrospective clinical evidence
Historically, there have been many small, retrospective studies investigating the clinical outcomes of second- or third-line line AR-axis-targeted therapies in patients with mCRPC following the failure of first-line therapy.12,13 Overall, clinical outcomes for new AR-axis therapies (abiraterone acetate plus prednisone, enzalutamide) appear to be similar,12 though there are no prospective phase III clinical trials directly comparing these agents.12 However, a pooled analysis of data from 10 of these studies (n=536; range of patients per study: 23–137) suggested there may be cumulative benefits in their sequential use.13 In addition, a retrospective chart review by Zhang et al. reported modest clinical activity as measured by a prostate-specific antigen (PSA) decline of ≥50% from baseline (PSA50) and progression-free survival (PFS; the time from initiation of therapy to first clinical/radiographic progression, death or last follow-up) in patients with mCRPC receiving enzalutamide (n=28) or docetaxel (n=13) who had previously received abiraterone acetate plus prednisone (with or without prior chemotherapy; Table 1).14 Similarly, a retrospective analysis conducted by Brasso et al. of 137 consecutive patients with mCRPC who received enzalutamide following taxane-based chemotherapy and abiraterone acetate plus prednisone also showed modest clinical improvements on PSA and OS (Table 1).15
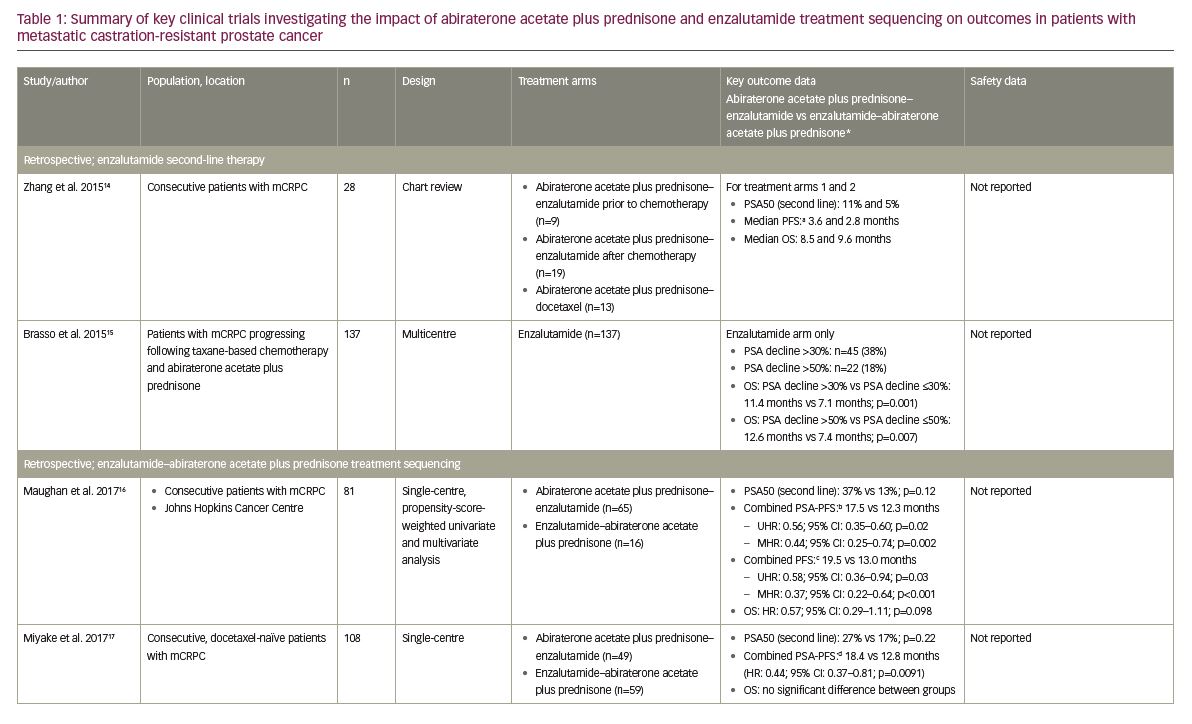
More recently, five retrospective studies have specifically investigated how the treatment sequencing of abiraterone acetate plus prednisone and enzalutamide may impact clinical outcomes in patients with mCRPC (Table 1), the results of which will be summarised here.10,16–19 Firstly, Maughan et al. conducted a single-centre, retrospective analysis of 81 consecutive patients with mCRPC treated with either abiraterone acetate plus prednisone–enzalutamide (n=65) or enzalutamide–abiraterone acetate plus prednisone (n=16) at the Johns Hopkins Cancer Centre, USA.16 Outcomes were adjusted for confounding factors using propensity score-weighted univariate and multivariate Cox analyses, and there were no significant differences in baseline characteristics between treatment groups.16 The median combined PSA-PFS (defined as the time from initiation of first therapy to a 25% increase in PSA on second therapy) was significantly longer in the abiraterone acetate plus prednisone–enzalutamide group versus the enzalutamide–abiraterone acetate plus prednisone group (17.5 versus 12.3 months; multivariate analysis hazard ratio [HR]: 0.44; 95% confidence interval [CI]: 0.26–0.74; p=0.002).16 The combined PFS (primary endpoint; the sum of the clinical/radiographic PFS on the first and second mCRPC agents) was also significantly longer when abiraterone acetate plus prednisone was administered first (19.5 versus 13.0 months, respectively; multivariate analysis HR: 0.37; 95% CI: 0.22–0.64; p<0.001).16 However, there was no significant difference in OS between treatment groups, although OS was numerically superior with abiraterone acetate plus prednisone–enzalutamide (HR: 0.57; 95% CI: 0.29–1.11, p=0.098).16
Miyake et al. conducted a retrospective analysis of 108 consecutive docetaxel-naïve patients with mCRPC treated with either abiraterone acetate plus prednisone–enzalutamide (n=49) or enzalutamide–abiraterone acetate plus prednisone (n=59) in Japan.17 No significant differences in baseline characteristics were noted between the two treatment groups.17 The PSA50 on second-line therapy was numerically, but not significantly, greater in the abiraterone acetate plus prednisone–enzalutamide group (26.5% versus 16.9%; p=0.22).17 However, the combined PSA-PFS (defined as the total combined time to PSA progression on each therapy) was significantly longer in the abiraterone acetate plus prednisone–enzalutamide group (18.4 versus 12.8 months; HR: 0.44; 95% CI: 0.37–0.81; p=0.0091).17 Consistent with the results of the study by Maughan et al., there was no significant difference in OS between the two treatment groups.16,17
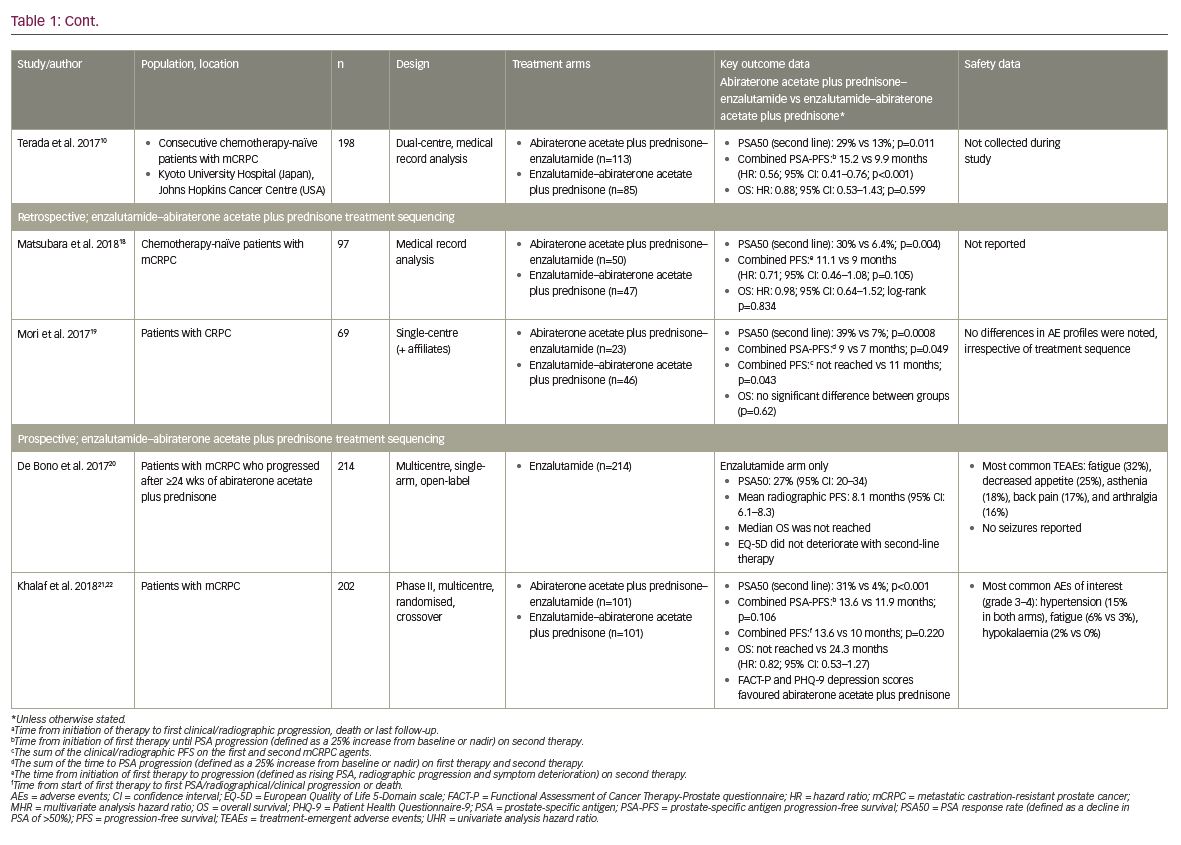
These study results were further supported by the findings of Terada et al., who conducted a retrospective analysis of data from 198 consecutive chemotherapy-naïve patients with mCRPC treated with either abiraterone acetate plus prednisone–enzalutamide (n=113) or enzalutamide–abiraterone acetate plus prednisone (n=85) at the Kyoto University Hospital, Japan, or Johns Hopkins Cancer Centre, USA.10 Some differences in baseline characteristics were noted between treatment groups, including the number of prior anti-androgen treatments, though there was no difference in Gleason score, the rate of bone or visceral metastatic disease, or baseline PSA.10 It should also be noted that the 58 pre-chemotherapy patients included in the Maughan et al. study were included in this analysis.10,16 Where Miyake et al. only reported a numerical difference in PSA50 response rate,17 results from this analysis showed that significantly more patients achieved PSA50 in the abiraterone acetate plus prednisone–enzalutamide group than the enzalutamide–abiraterone acetate plus prednisone group (29% versus 13%; p=0.011).10 Also, the combined PSA-PFS (defined as per Maughan et al. 201716) was again significantly longer in the abiraterone acetate plus prednisone–enzalutamide group compared with the enzalutamide–abiraterone acetate plus prednisone group (15.2 versus 9.9 months; univariate HR: 0.56; 95% CI: 0.41–0.76; p<0.001). This difference was also found to be significant in multivariate analyses (HR: 0.65; 95% CI: 0.42–0.99; p=0.044).10 Consistent with previous studies, there was no significant difference in OS between treatment groups in either univariate or multivariate analyses (HR: 0.88; 95% CI: 0.53–1.43; p=0.599 and HR: 0.81; 95% CI: 0.49–1.35; p=0.427, respectively).10
Most recently, studies in Japan by Matsubara et al. and Mori et al. have provided further evidence to support these initial findings.18,19 Matsubara et al. conducted a retrospective medical record analysis of 97 chemotherapy-naïve patients with mCRPC treated with either abiraterone acetate plus prednisone–enzalutamide (n=50) or enzalutamide–abiraterone acetate plus prednisone (n=47).18 Patient characteristics at the time of initiation of first-line and second-line therapies were generally balanced, though median baseline PSA values were significantly greater in the enzalutamide–abiraterone acetate plus prednisone group at initiation of first-line therapy (33.1 versus 52.0 ng/mL; p=0.037).18 Similar to the data presented by Terada et al.,10 significantly more patients achieved PSA50 for second-line therapy in the abiraterone acetate plus prednisone–enzalutamide group compared with the enzalutamide–abiraterone acetate plus prednisone group (30% versus 6.4%; p=0.004).18 However, the combined PFS (time from start of first-line therapy to progression [defined as rising PSA, radiographic progression and symptom deterioration] on second-line therapy) was only numerically, not significantly, longer in the abiraterone acetate plus prednisone–enzalutamide group (11.1 versus 9.0 months; HR: 0.71; 95% CI: 0.46–1.08; p=0.105).18 Also, the order of treatment had no significant impact on OS (HR: 0.98; 95% CI: 0.64–1.52; log-rank p=0.834).18
Overall, with the exception of second-line PSA response, there were no significant differences in clinical outcomes between treatment sequencing groups.18 However, more consistent findings were reported by Mori et al., who conducted a retrospective analysis of 69 patients with CRPC treated with either abiraterone acetate plus prednisone–enzalutamide (n=23) or enzalutamide–abiraterone acetate plus prednisone (n=46).19 Baseline characteristics were similar between treatment groups, with the exception of haemoglobin values.19 Significantly more patients achieved PSA50 for second-line therapy in the abiraterone acetate plus prednisone–enzalutamide group compared with the enzalutamide–abiraterone acetate plus prednisone group (39.1% versus 6.5%; p=0.0008).19 In addition, the combined PSA-PFS (sum of the time to PSA progression [25% increase from baseline or nadir] on both therapies) was significantly longer in the abiraterone acetate plus prednisone–enzalutamide group (9 versus 7 months; p=0.049),19 and the combined PFS (combined time to symptomatic or radiographic progression on both therapies) was significantly longer in the abiraterone acetate plus prednisone–enzalutamide group (not reached versus 11 months; p=0.043).19 Consistent with all other retrospective studies, OS was not significantly different between treatment groups (p=0.63).19
Overall, clinical outcomes across the five retrospective studies consistently favoured abiraterone acetate plus prednisone–enzalutamide over enzalutamide–abiraterone acetate plus prednisone in patients with mCRPC.10,16–19 These included PSA50 (for second-line therapy; 26.5–39.1% versus 6.4–16.9%),10,16–19 combined PSA-PFS (9–18.4 months versus 7–12.8 months)10,16,17,19 and combined PFS (≥11.1 versus 9–13 months, respectively).10,16,19 However, no significant differences in OS between treatment sequencing groups were observed in any of the five retrospective studies.10,16–19
Treatment sequencing – prospective clinical evidence
The results of the five retrospective studies detailed earlier helped to generate the hypothesis that sequencing abiraterone acetate plus prednisone before enzalutamide may provide efficacy benefits in patients with mCRPC compared with sequencing vice versa.10,16–19 However, as with many retrospective studies, there were potential confounding effects, including differences in study designs, clinical outcomes, outcome definitions, baseline characteristics, treatment decisions and safety monitoring.10,16–19 As such, further evidence from more robust, prospective clinical trials was required to validate the hypothesis. To date, two prospective studies have been conducted, which both show similar efficacy trends to those observed in the retrospective studies.20–22
Firstly, De Bono et al. conducted a prospective, multicentre, single-arm, open-label phase IV study to evaluate the efficacy and safety of enzalutamide (160 mg/day, administered orally) in 214 patients with mCRPC who had progressed following ≥24 weeks of treatment with abiraterone acetate plus prednisone.20 While prior chemotherapy was permitted, it was not required for study entry; demographics and baseline characteristics were generally similar between patients who had received prior chemotherapy before abiraterone acetate plus prednisone (n=69) and those who were chemotherapy-naïve (n=145).20 Overall, 27% (95% CI: 20–34) of patients achieved PSA50 with enzalutamide second-line therapy following abiraterone acetate plus prednisone first-line therapy, with a median radiographic PFS duration of 8.1 months (95% CI: 6.1–8.3; PFS was defined as the time from enzalutamide therapy to objective evidence of radiographic disease progression [including ≥2 new lesions for bone disease progression]).20 The median OS was not reached by the time of the data cut-off point due to the low number of events reported.20
Patient health-related quality of life (QoL) was also assessed using the European Quality of Life 5-Domain scale (EQ-5D).23 Overall, patient QoL did not deteriorate during second-line treatment with enzalutamide, with EQ-5D visual analogue scores remaining above 70 up to week 49 of the study.20 With regard to safety outcomes, adverse events were consistent with the known safety profile of enzalutamide.20 Overall, 93% of patients experienced a treatment-emergent adverse event, the most common of which were fatigue (32%), decreased appetite (25%), asthenia (18%), back pain (17%), and arthralgia (16%).20 Eighty-one patients (38%) experienced a serious adverse event, though no seizures were reported.20 Of the 19 (9%) fatal treatment-emergent adverse events, only one (cerebral infarction) was considered possibly related to the study drug.20
Secondly, Khalaf et al. conducted a prospective, multi centre phase II trial in 202 patients with mCRPC treated with either abiraterone acetate plus prednisone–enzalutamide (n=101) or enzalutamide–abiraterone acetate plus prednisone (n=101).21 Baseline characteristics at the time of second-line therapy were generally balanced; differences between the abiraterone acetate plus prednisone–enzalutamide and enzalutamide–abiraterone acetate plus prednisone groups included Eastern Co-operative Oncology Group score (89% versus 76% with scores of 0–1, respectively; p=0.044) and serum lactate dehydrogenase levels (more than the upper limit of normal in 25% versus 8% of patients; p=0.013).21 Following second-line therapy, significantly more patients had achieved PSA50 in the abiraterone acetate plus prednisone–enzalutamide group compared with the enzalutamide–abiraterone acetate plus prednisone group (31% versus 4%; p<0.001). In addition, the median time to a second PSA progression (from combined first- and second-line therapy) and overall progression (PSA, radiographic, clinical progression or death) were numerically, but not significantly, longer in the abiraterone acetate plus prednisone–enzalutamide group compared with the enzalutamide–abiraterone acetate plus prednisone group (13.6 versus 11.9 months; p=0.106 and 13.6 versus 10 months; p=0.220, respectively).21 The median OS was not reached in the abiraterone acetate plus prednisone–enzalutamide group, but was 24.3 months in the enzalutamide–abiraterone acetate plus prednisone group (HR: 0.82; 95% CI: 0.53–1.27).21 Patient QoL was also assessed using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) and Patient Health Questionnaire-9 (PHQ-9) questionnaires.22 For first-line treatment, patient-reported outcomes favoured abiraterone acetate plus prednisone compared with enzalutamide for both FACT-P (p=0.003 in patients ≥75 years of age) and PHQ-9 depression scores, but as these were not pre-specified analyses the results should be considered hypothesis-generating.22
In summary, clinical outcomes across both prospective studies showed benefits for abiraterone acetate plus prednisone–enzalutamide treatment sequencing versus enzalutamide–abiraterone acetate plus prednisone sequencing in patients with mCRPC on PSA50 during second line therapy (27–31% versus 4%),20,21 time to second PSA progression (13.6 versus 11.9 months),21 and time to second disease progression (13.6 versus 10 months).21 However, no significant impact was observed on OS.21
Discussion
Currently, there is no expert consensus on the use of second-line treatment with abiraterone acetate plus prednisone or enzalutamide in patients with mCRPC who have progressed on either treatment, due in part to the paucity of prospective clinical data in these settings.20 However, the results of recent clinical trials suggests that the sequencing of new AR-axis-targeted therapies may impact clinical outcomes in these patients.10,16–21 Overall, the balance of evidence from five retrospective and two prospective clinical trials, enrolling a total of 911 patients with mCRPC who had failed first-line treatment strategies, suggests that an abiraterone acetate plus prednisone–enzalutamide sequence may provide more favourable combined PSA-PFS and/or combined PFS outcomes versus enzalutamide–abiraterone acetate plus prednisone.10,16–21 As yet, there is no clearly-defined molecular mechanism that can explain the apparent benefits of an abiraterone acetate plus prednisone–enzalutamide treatment sequence over enzalutamide–abiraterone acetate plus prednisone; a better understanding of the biology of abiraterone acetate plus prednisone and enzalutamide resistance is therefore key to optimising the use of these agents.20
In contrast to PSA and PFS outcomes, no significant differences were observed in OS, though the small sample sizes and low mortality rates suggest that the studies were not sufficiently powered to detect differences in this outcome. Indeed, the median OS was not reached in the abiraterone acetate plus prednisone–enzalutamide group in either of the prospective studies, highlighting the need for larger and more appropriately-designed prospective trials to confirm these findings.20,21 Significant differences in key baseline characteristics were also reported in some of the retrospective studies (including the number and type of prior anti-androgen therapies),10 again making the detection of significant between-group differences in OS challenging.
There are several additional limitations to be considered when interpreting the results of these studies. Firstly, most were retrospective, non-randomised studies with a lack of appropriate controls and differences not only in the primary outcomes assessed, but also in the definitions of PSA and PFS themselves. Secondly, there was heterogeneity in the timing and the rationale for switching between treatment sequences, especially in the retrospective studies where there were often no defined criteria for switching (e.g., radiographic criteria or PSA progression). Thirdly, data on adverse events and dose reductions/interruptions were not often collected, meaning that the impact of tolerability and adherence on clinical outcomes could not be assessed. Lastly, there was an absence of biomarker data in many studies (e.g., haemoglobin, alkaline phosphatase, lactate dehydrogenase), which may have provided further insight into the potential differences between treatments, prognostic factors and clinical outcomes.
Conclusions
In conclusion, the balance of evidence from recent clinical studies suggests that sequencing abiraterone acetate plus prednisone before enzalutamide as first-line therapy for mCRPC may provide greater efficacy on PSA50, PSA-PFS, and PFS than sequencing enzalutamide before abiraterone acetate plus prednisone. However, because of the lack of OS difference in all of these studies, both sequencing strategies are currently supported by the evidence. 







