touchCONGRESS Advances in the role of BCMA-targeting agents in multiple myeloma: An update from the EHA Congress 2021
Watch this two-part activity exploring recent data for BCMA-targeting agents in multiple myeloma. Filmed following the European Hematology Association 2021 Virtual Congress.
Part 1: Watch multiple myeloma expert Dr María-Victoria Mateos review key data from the EHA2021 Virtual Congress Watch Now
Part 2: Choose from leading experts who discuss what these data may mean for global and regional practice Select An Interview
Introduction
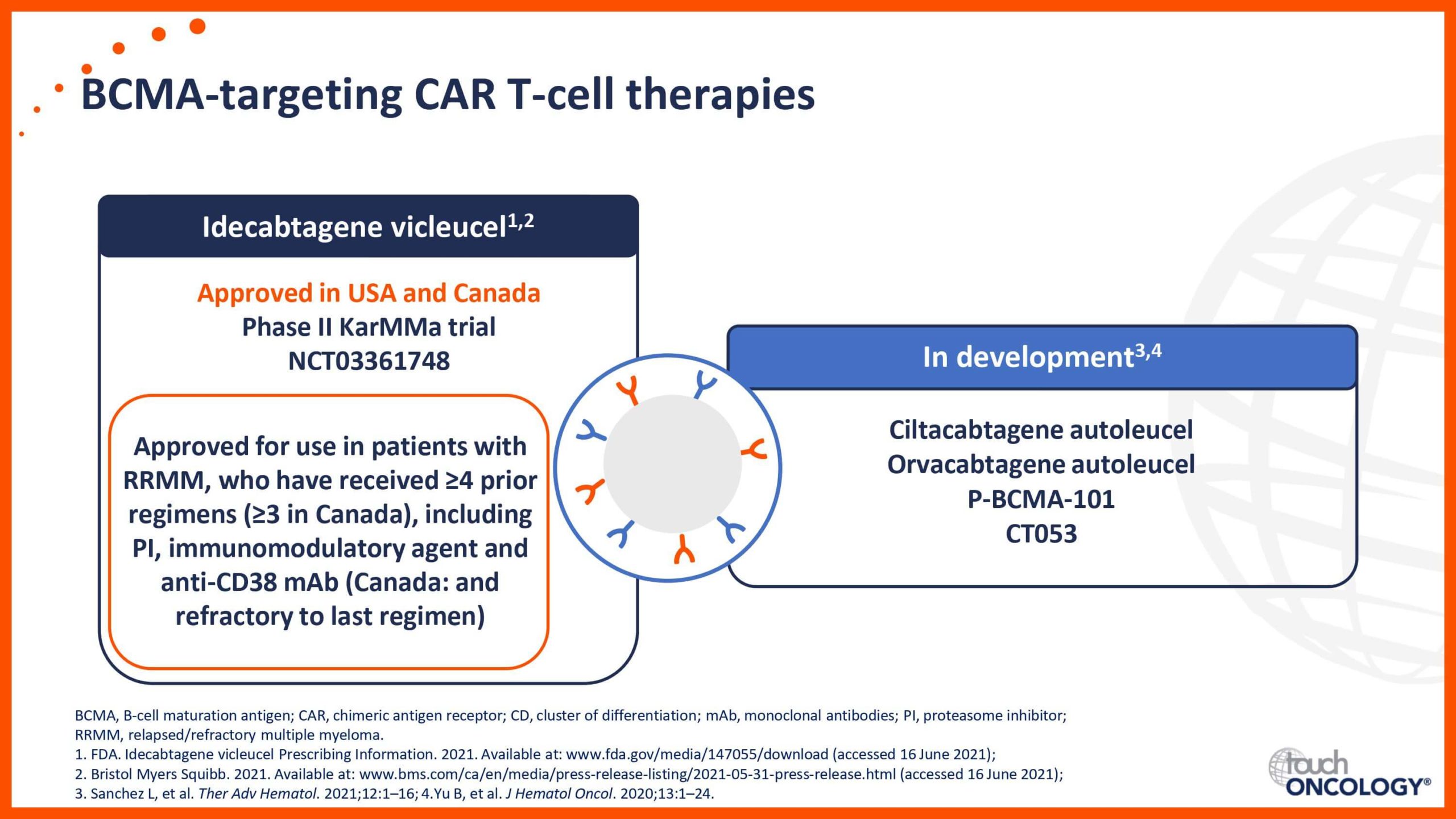
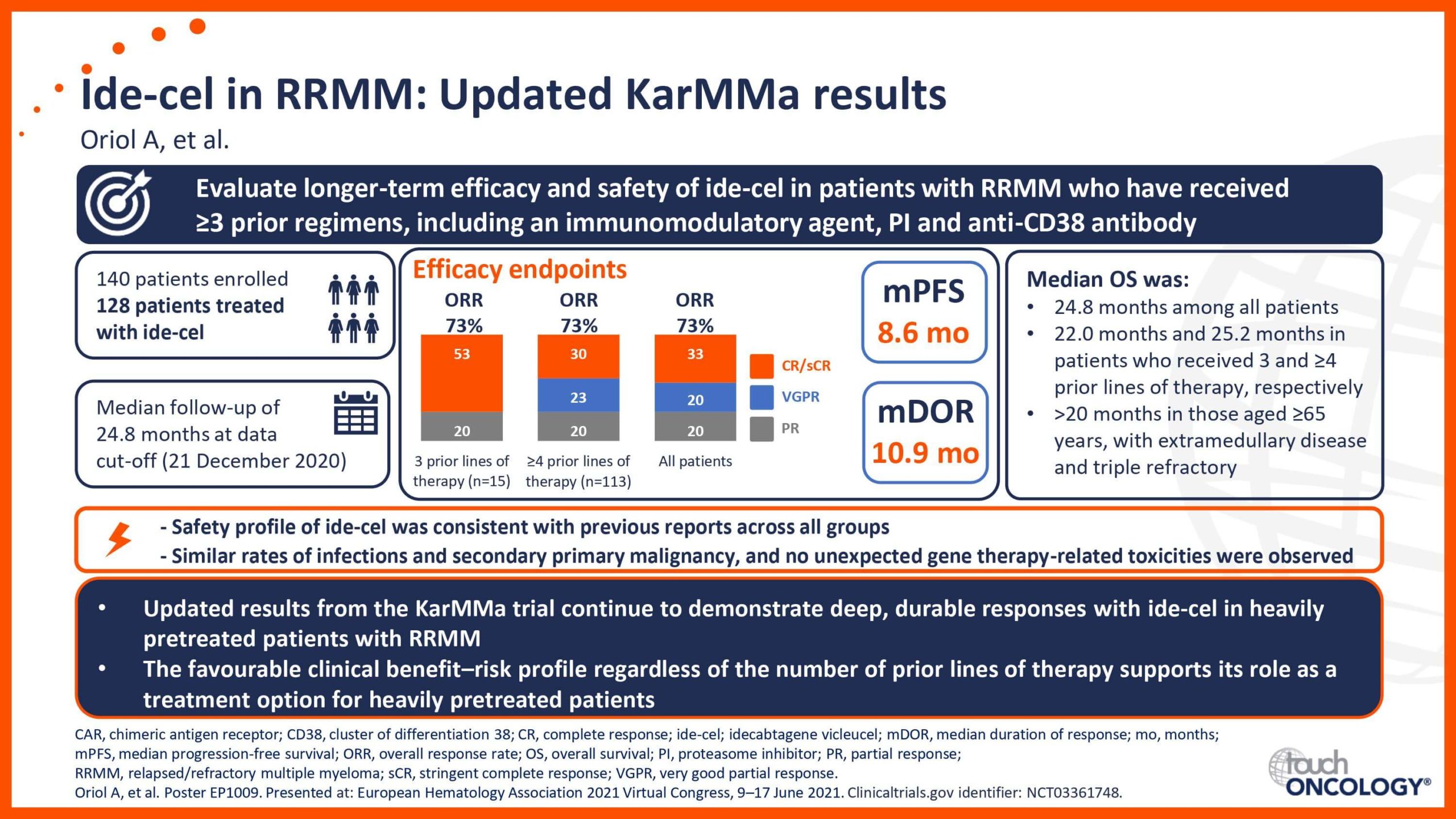
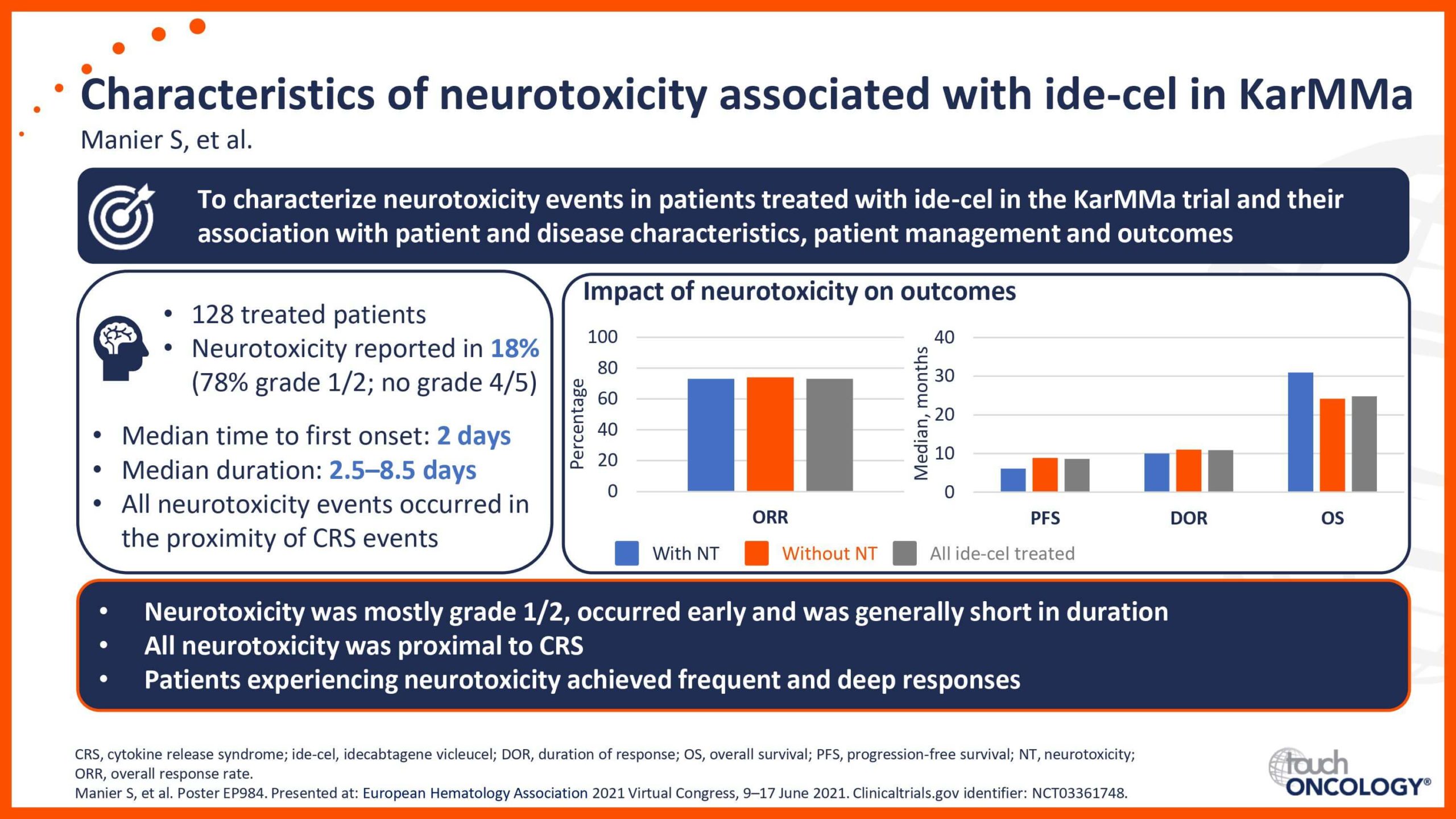
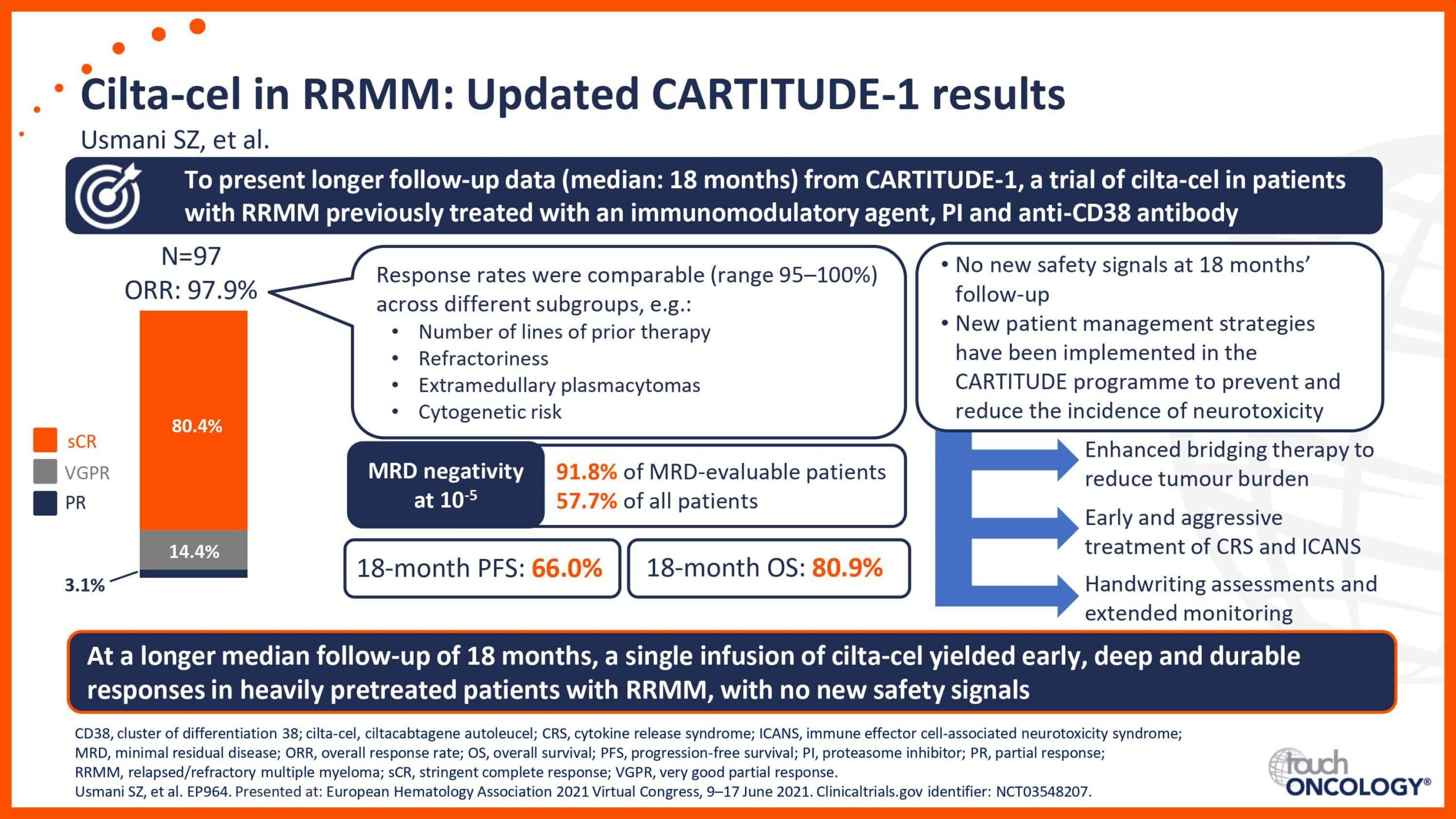
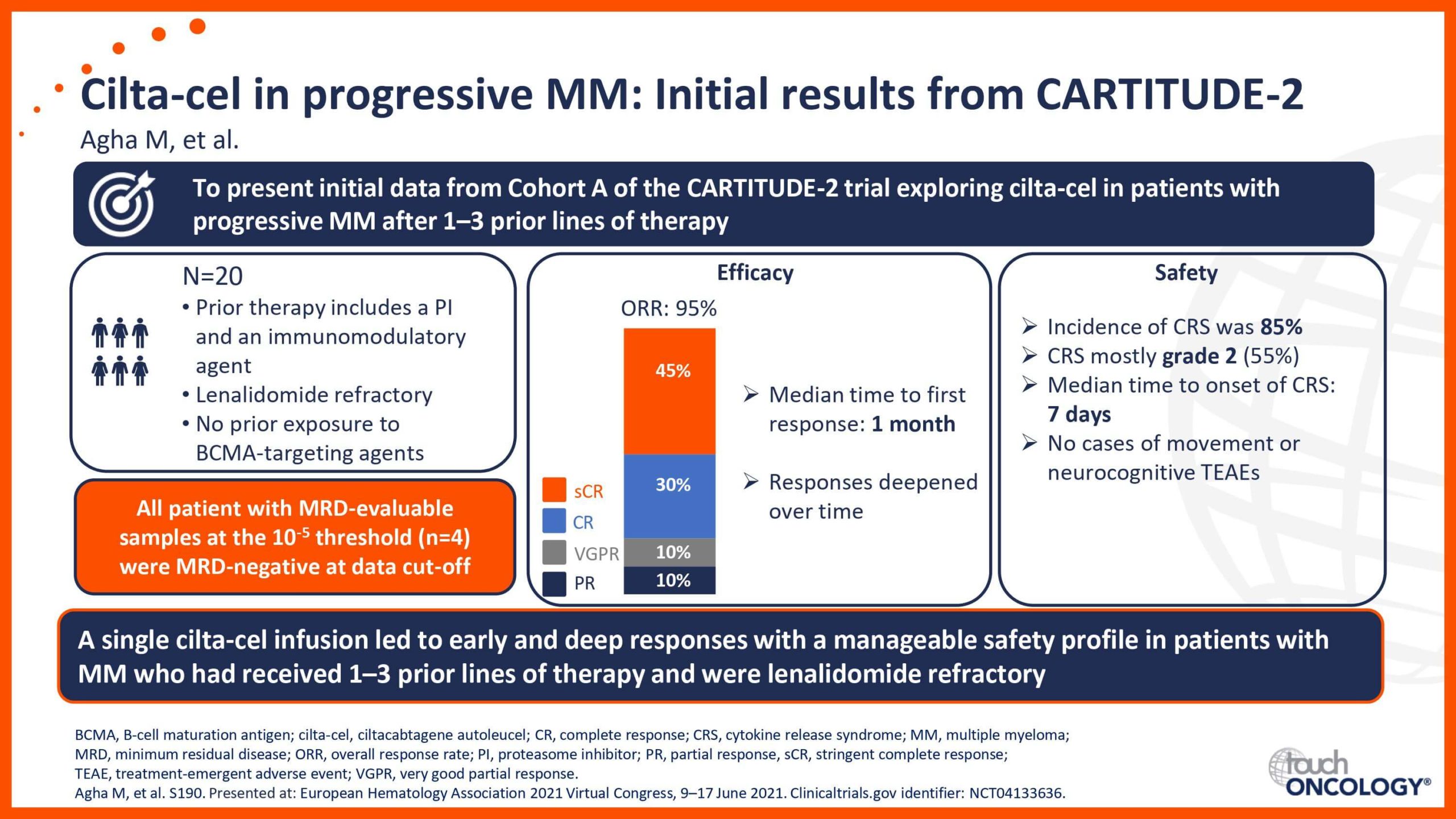
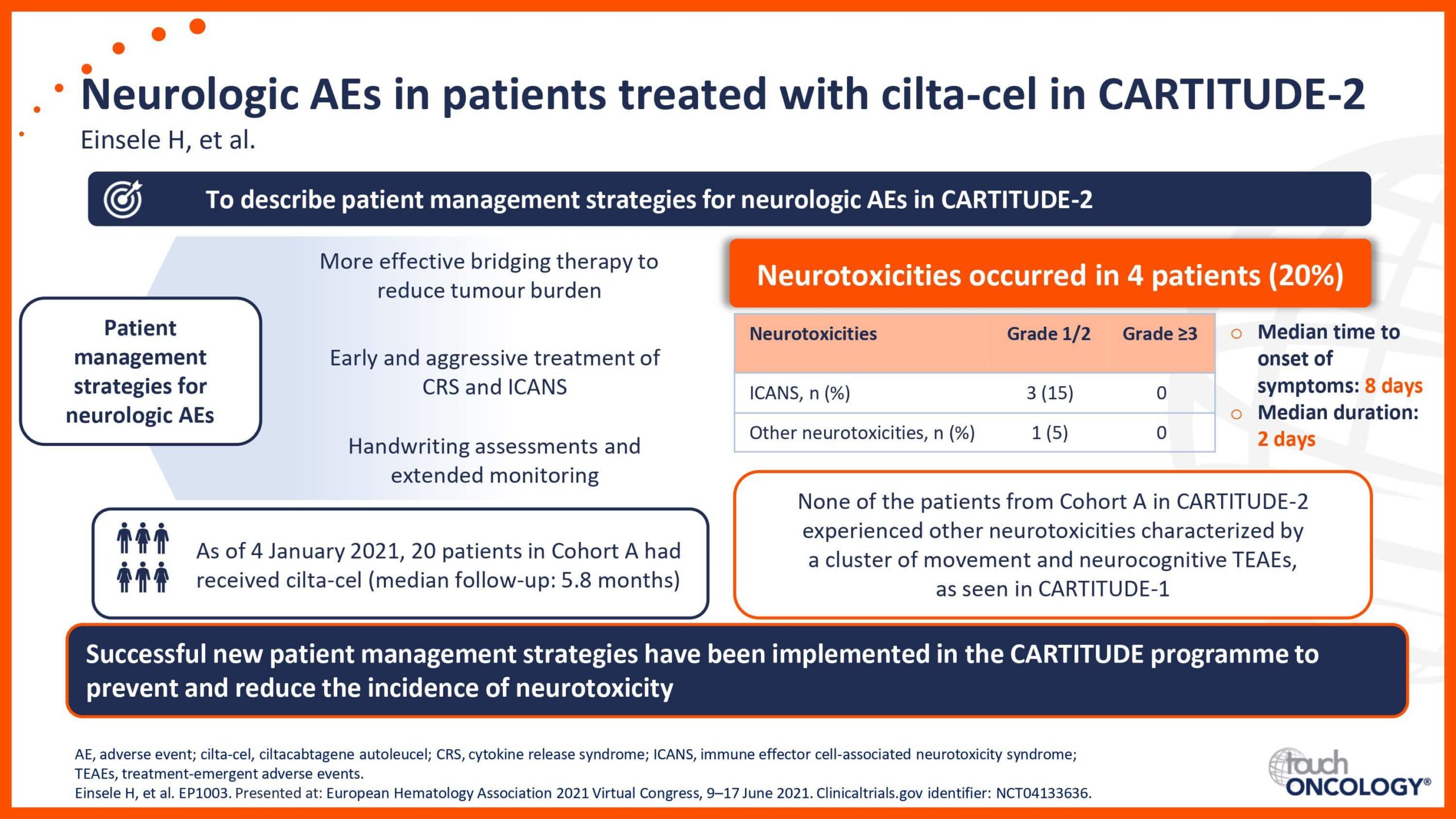
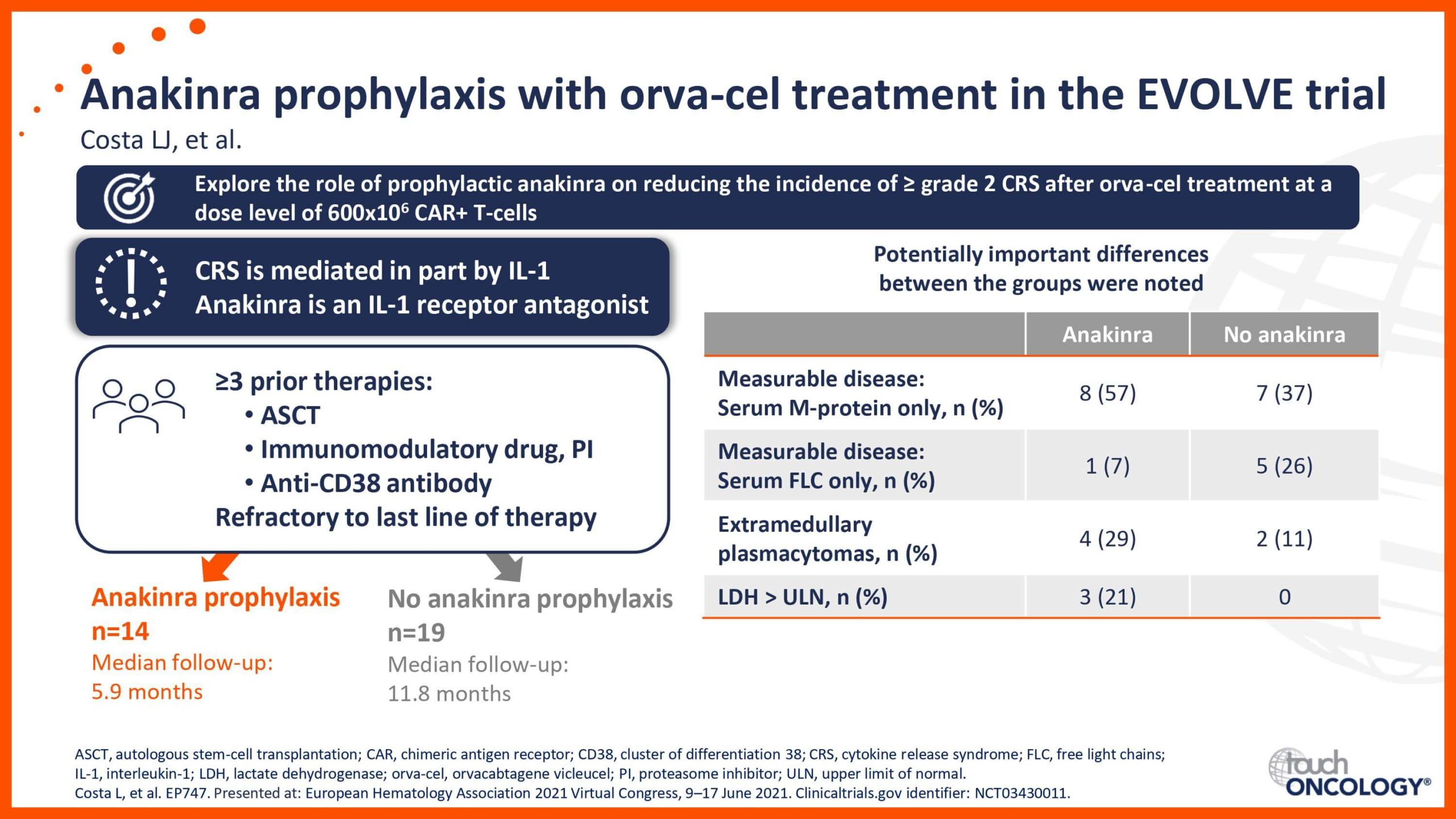
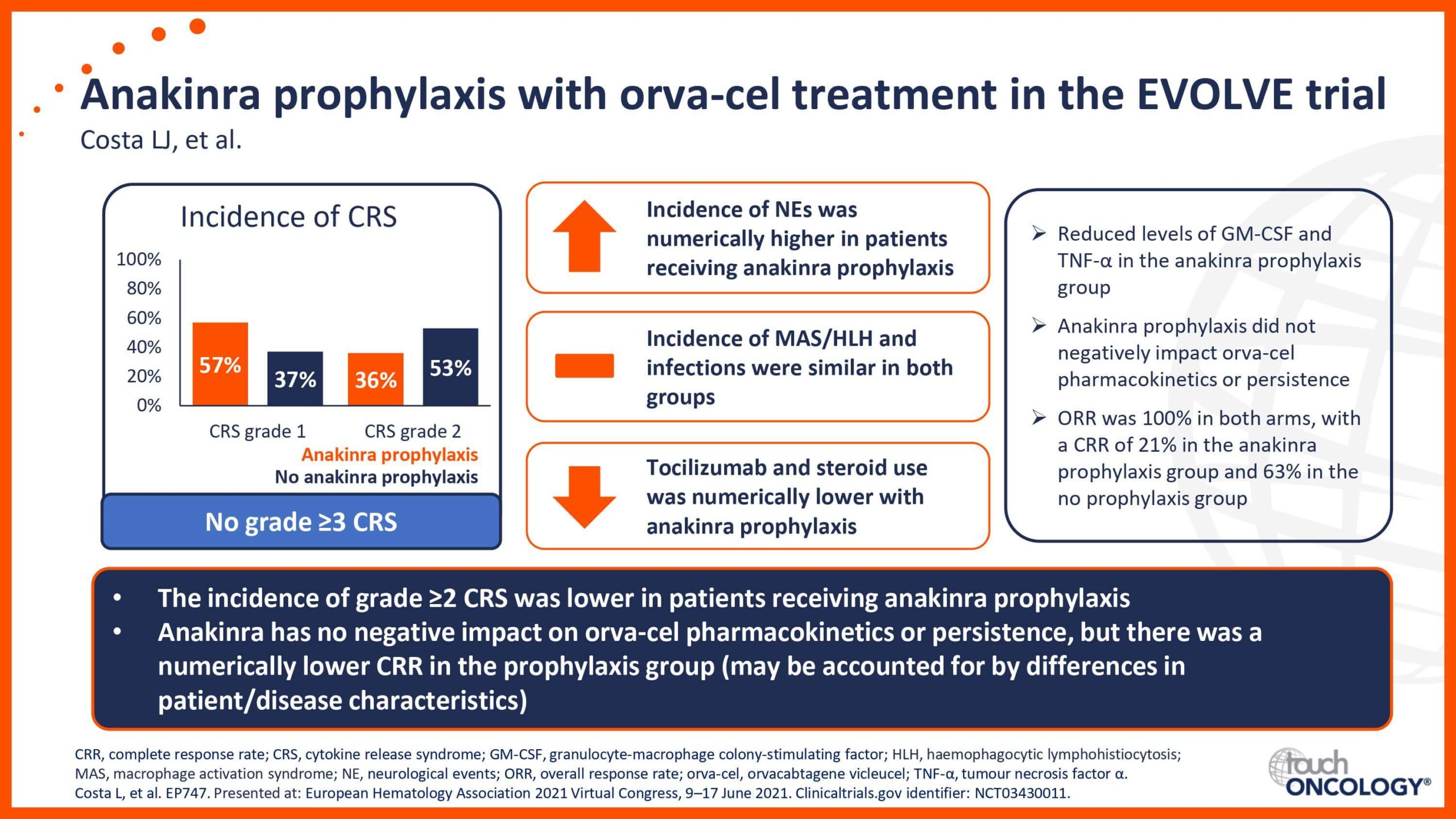
BCMA-targeting CAR T-cell therapies in relapsed/refractory multiple myeloma
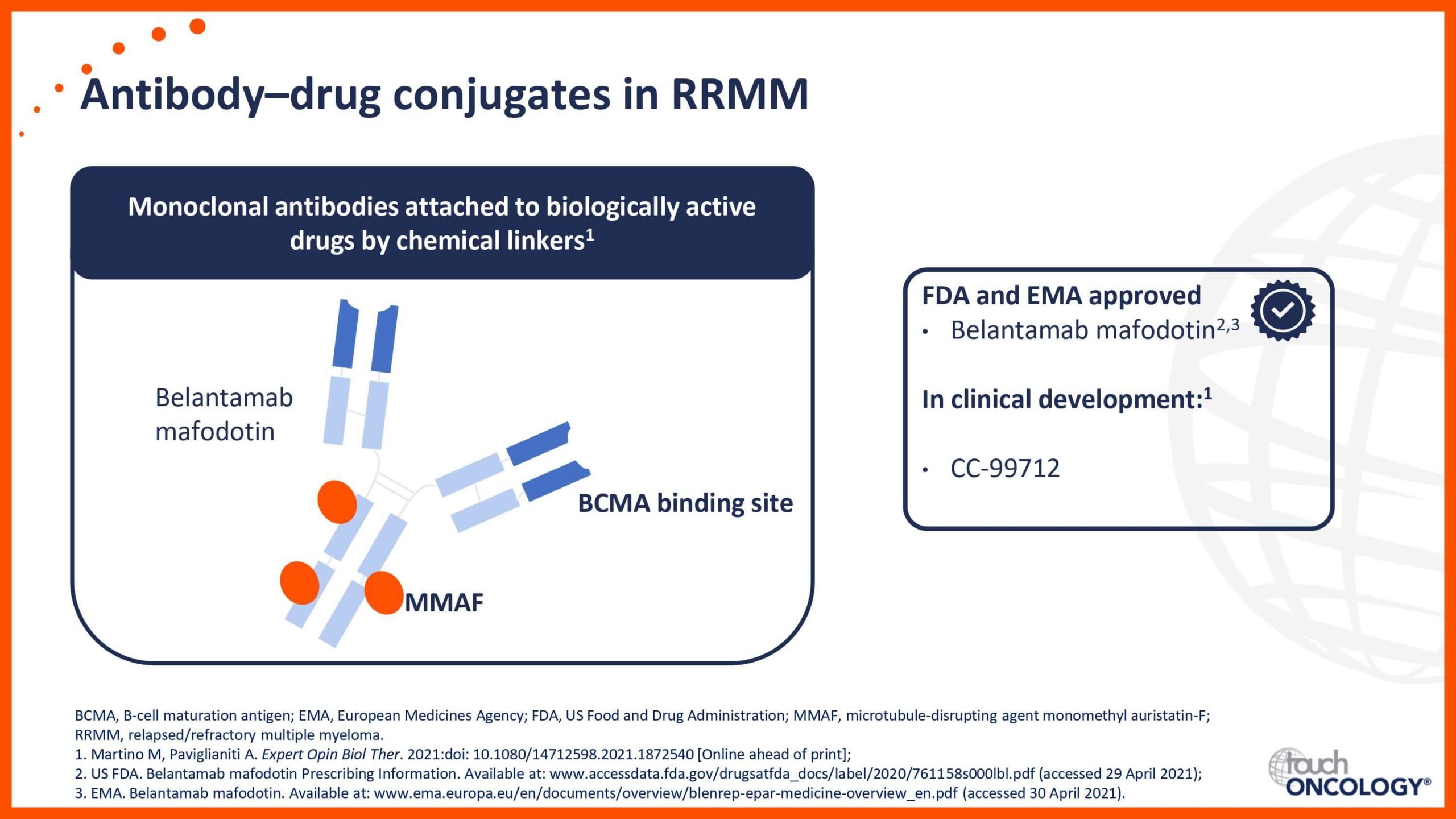
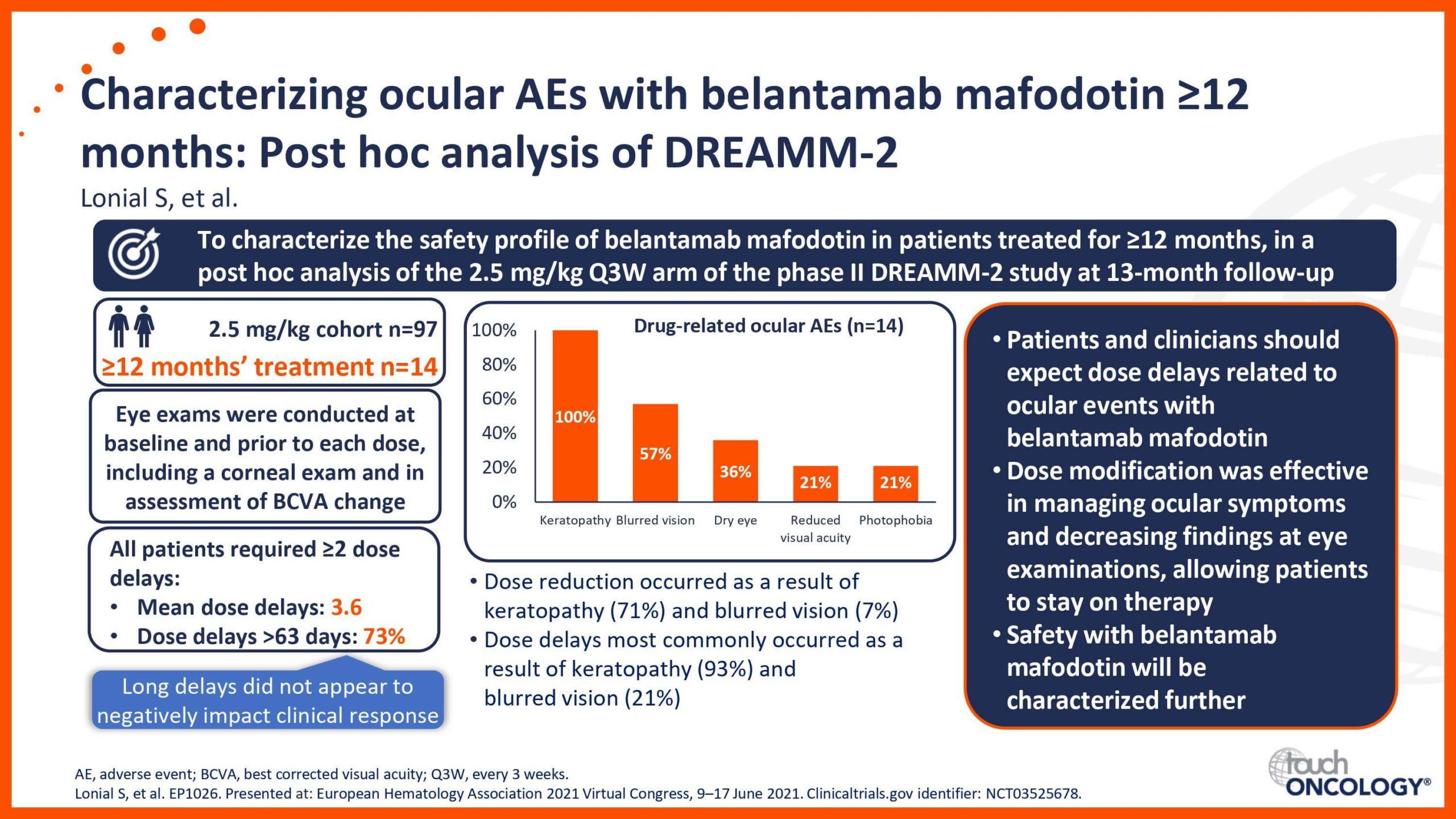
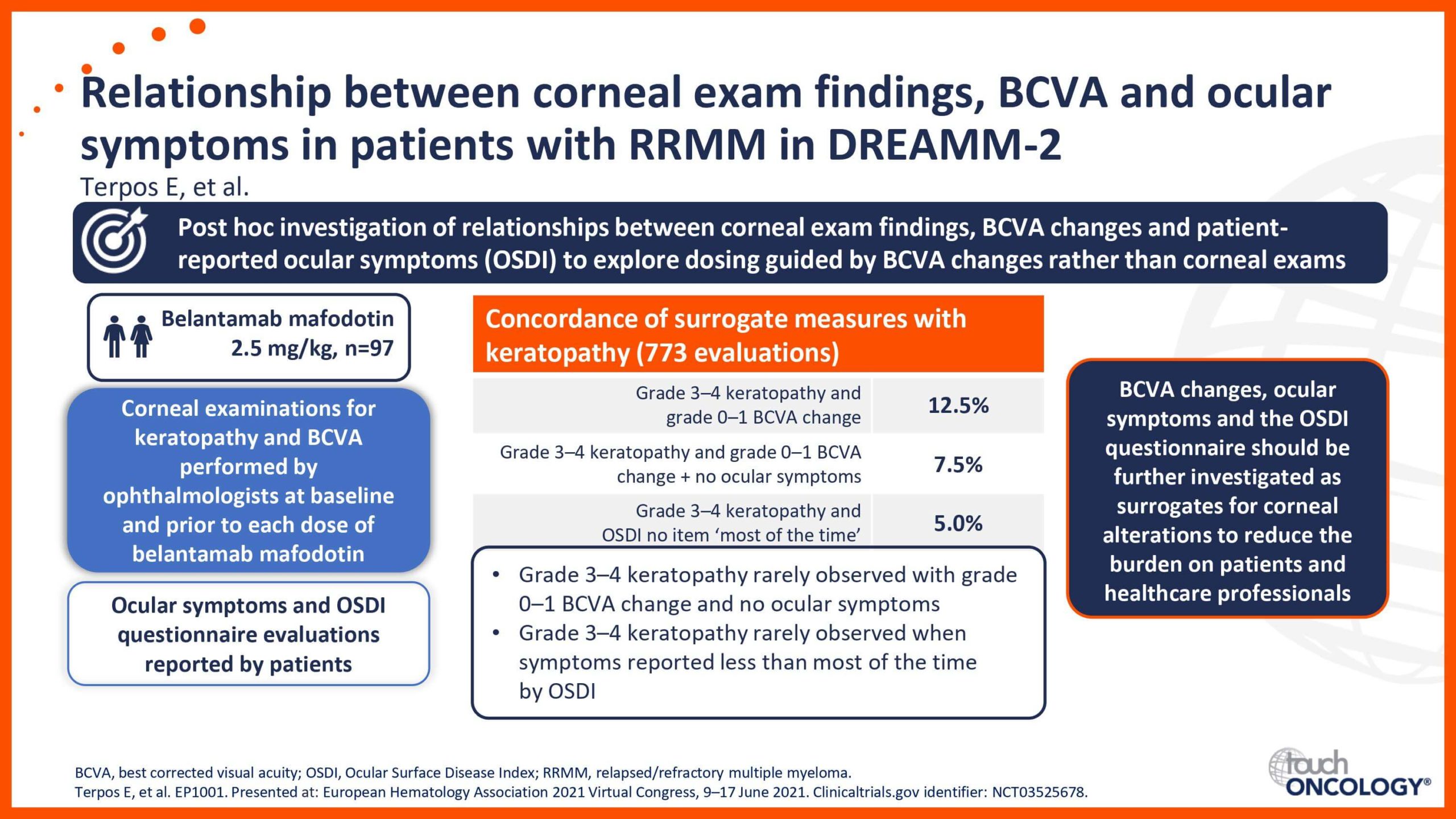
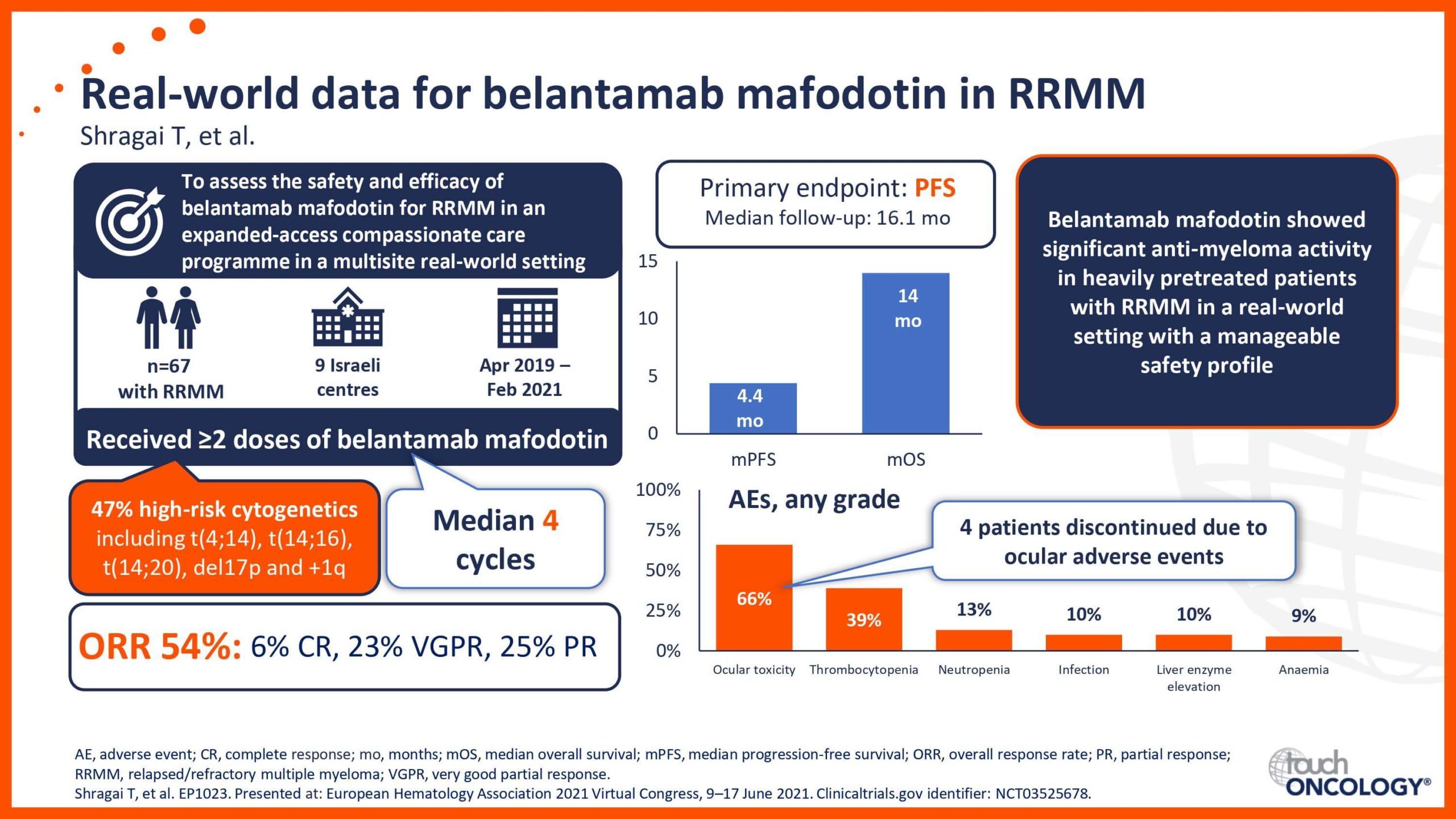
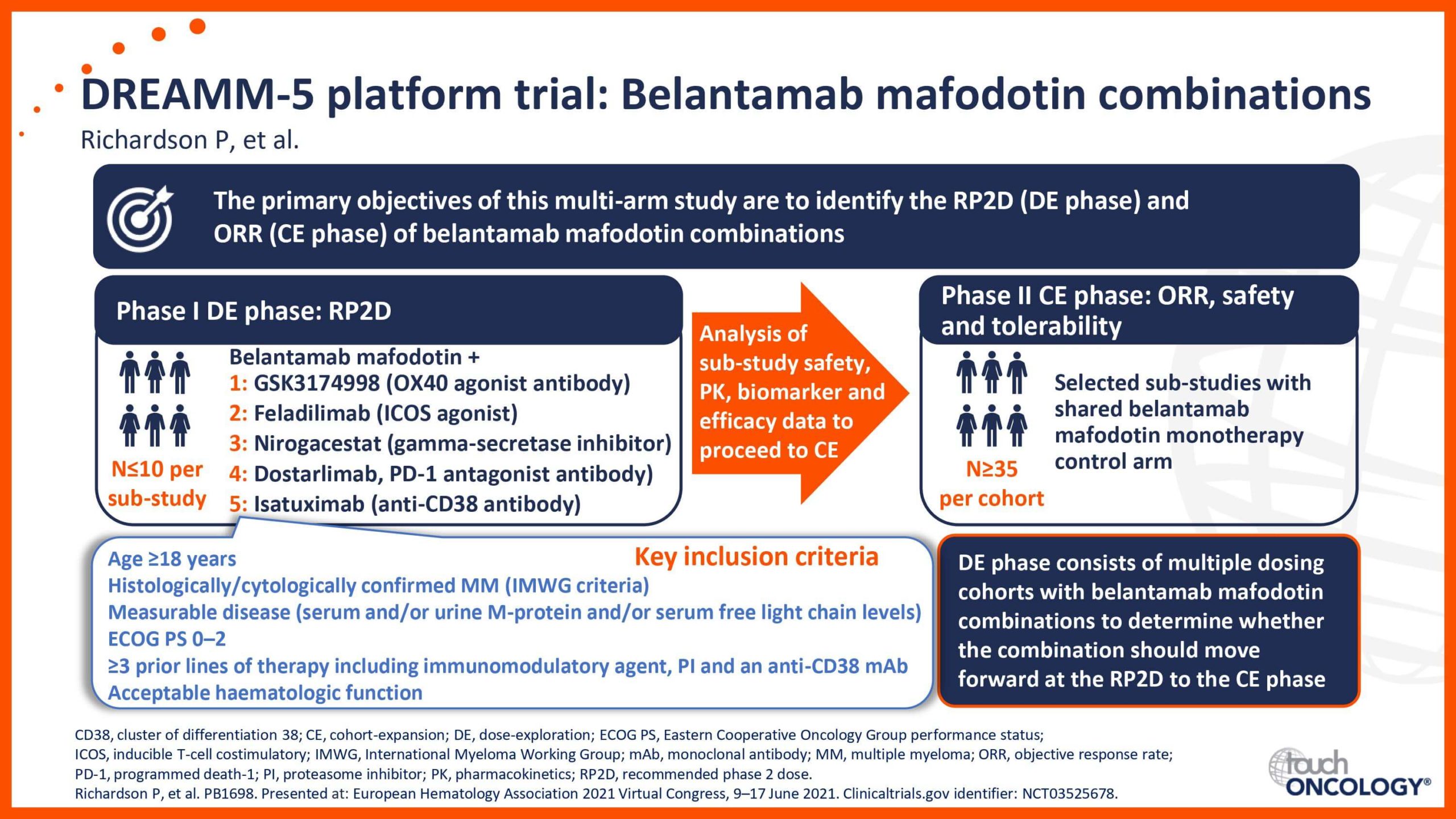
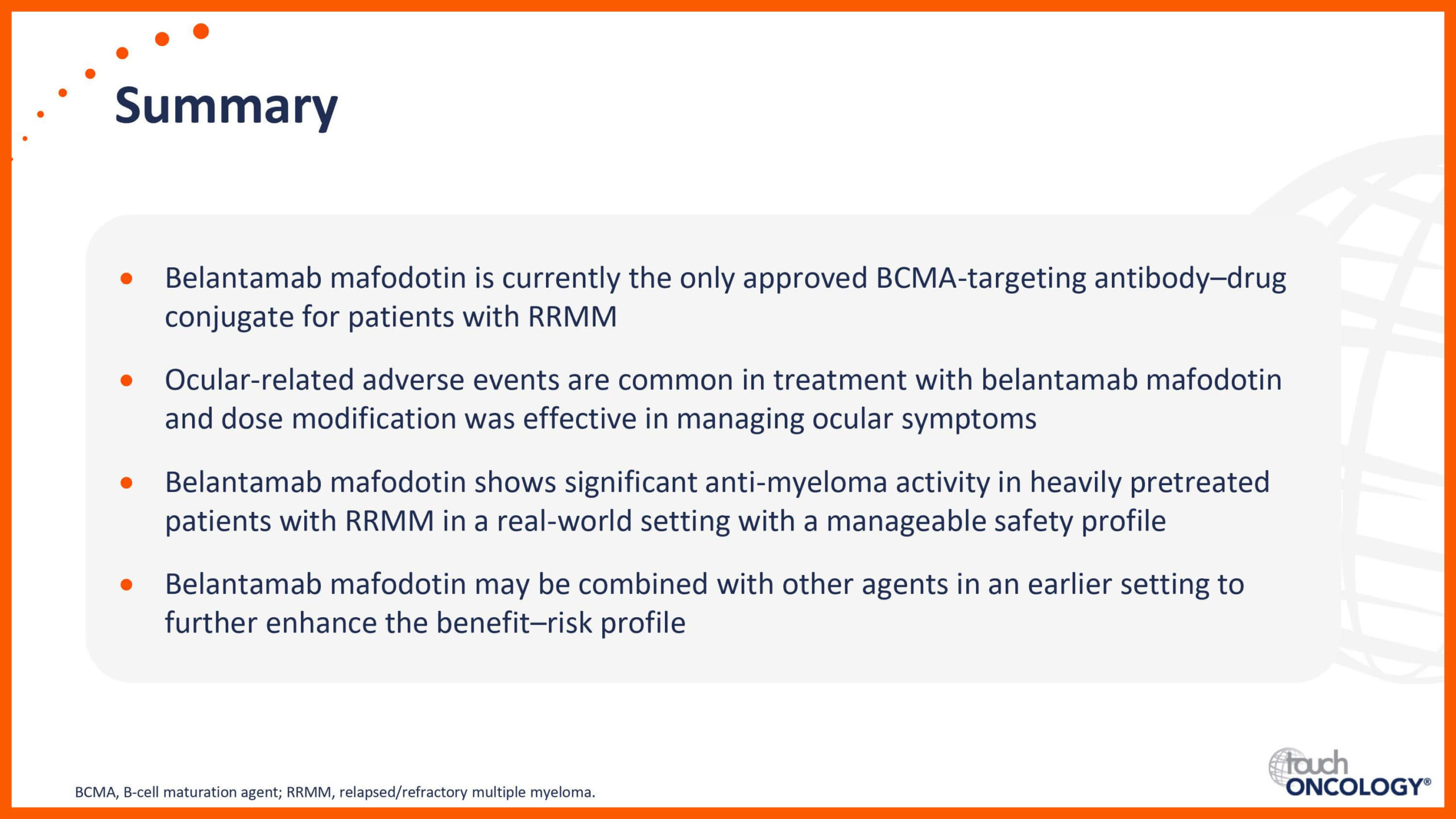
BCMA-targeting antibody–drug conjugates in relapsed/refractory multiple myeloma
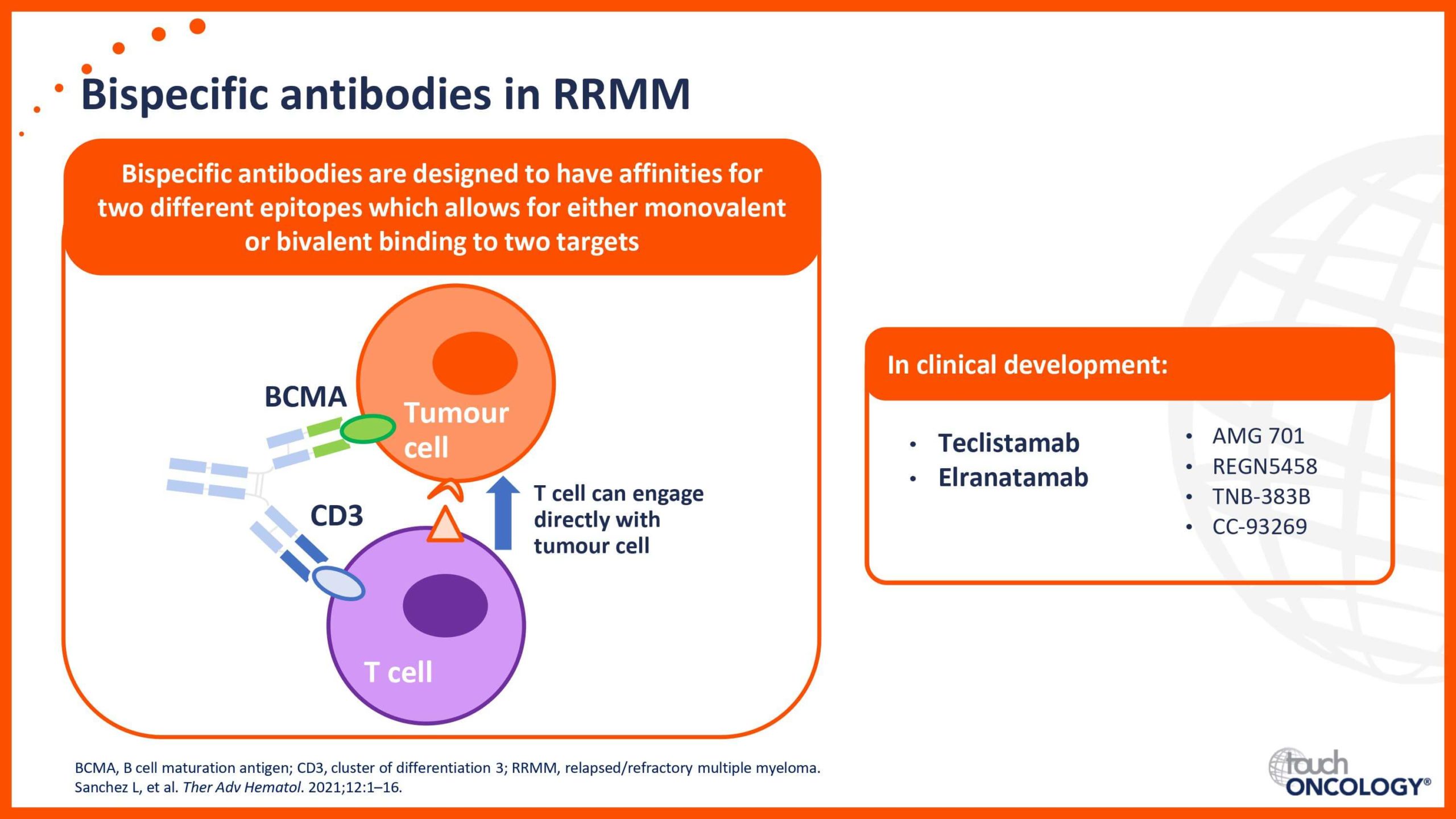
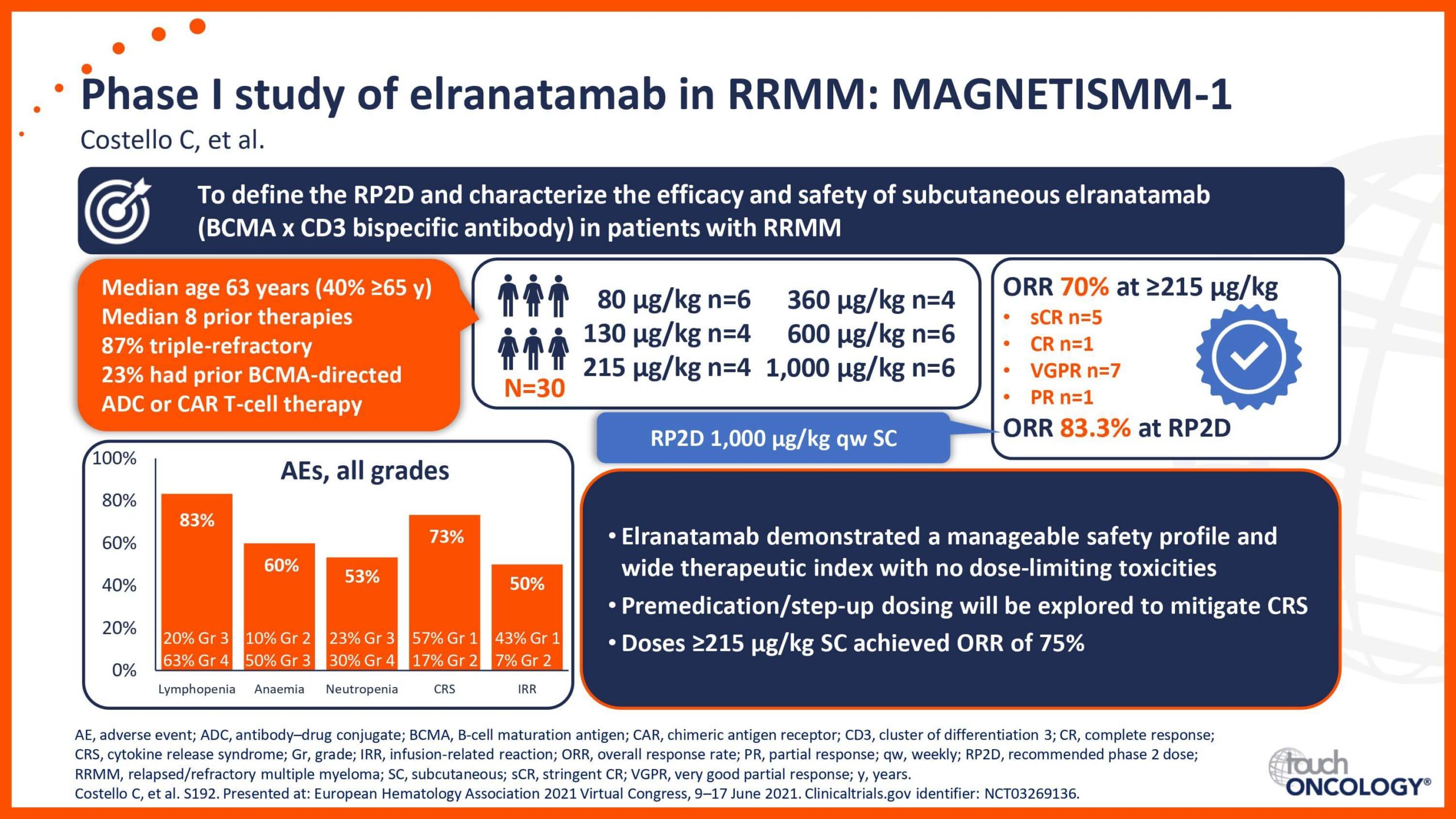
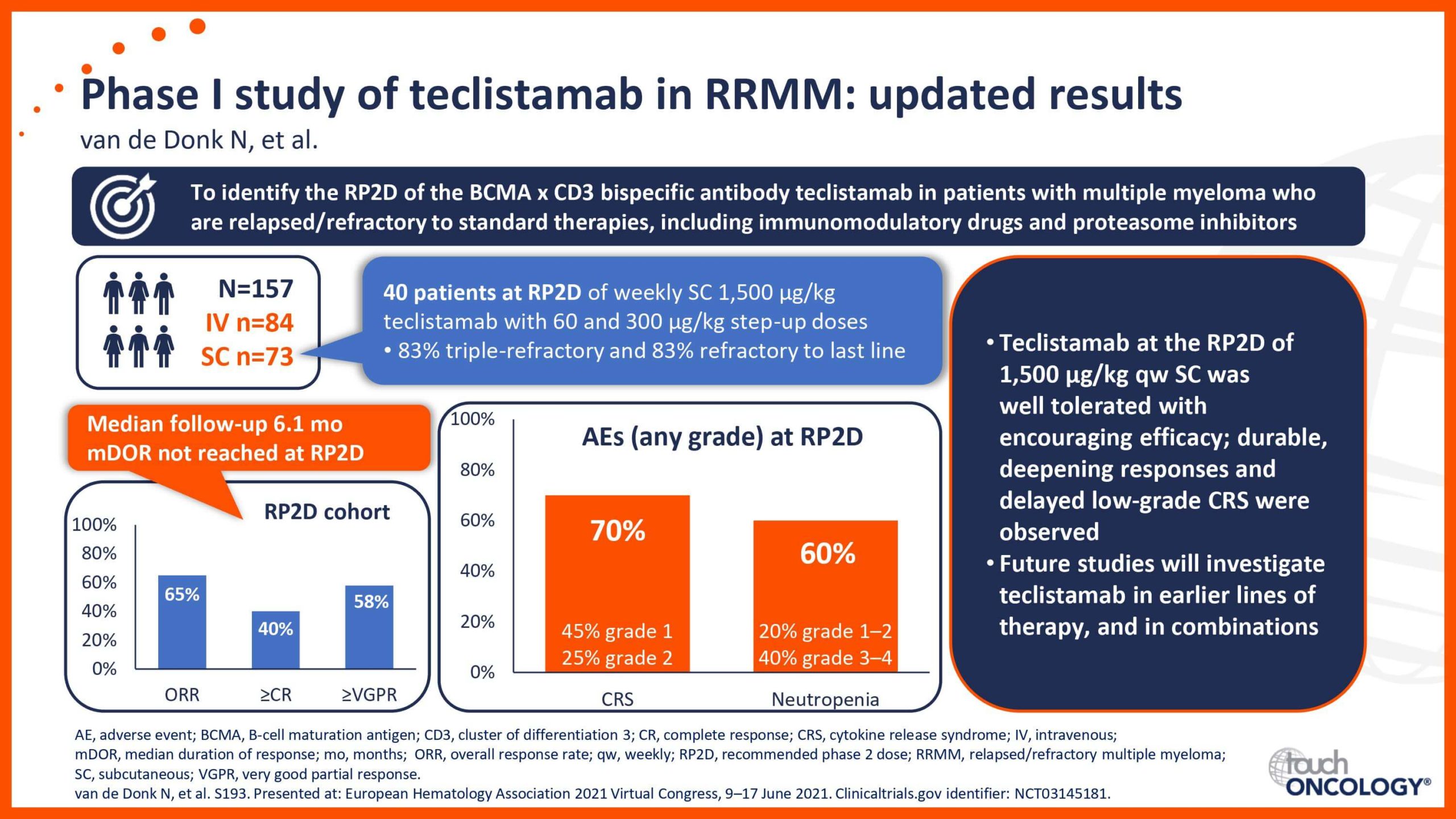
BCMA-targeting bispecific antibodies in relapsed/refractory multiple myeloma
María-Victoria Mateos considers the latest data on BCMA-targeting agents for the management of relapsed/refractory multiple myeloma from the EHA2021 Virtual Congress.
Heather Sutherland considers the latest data on BCMA-targeting agents for the management of relapsed/refractory multiple myeloma from the EHA2021 Virtual Congress.
Niels van de Donk considers the latest data on BCMA-targeting agents for the management of relapsed/refractory multiple myeloma from the EHA2021 Virtual Congress.
Please Select A Video:
Overview & Learning Objectives
Overview
Stay up to date with the latest data for BCMA-targeting agents in the treatment of multiple myeloma in this two-part activity. Filmed following the EHA2021 Virtual Congress.
Learning Objectives
After watching this activity, participants should be better able to:
- Recall efficacy data for BCMA-targeting agents in the treatment of multiple myeloma
- Describe the safety data for BCMA-targeting agents in the treatment of multiple myeloma
- Discuss how the latest data on BCMA-targeting agents may affect the management of patients with multiple myeloma

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights