touchCONGRESS HR+/HER2- Advanced breast cancer: what are the latest developments in CDK4/6 inhibition from the ASCO Meeting 2019?
Stay up-to-date with the latest developments in CDK4/6 inhibition for advanced breast cancer with our expert summary from the American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, United States, 31 May–4 June 2019.
Part 1: Watch internationally renowned Dr Joyce A. O’Shaughnessy review key data from ASCO 2019
Part 2: Choose from leading experts who discuss what the data findings mean for global and regional practice
Introduction
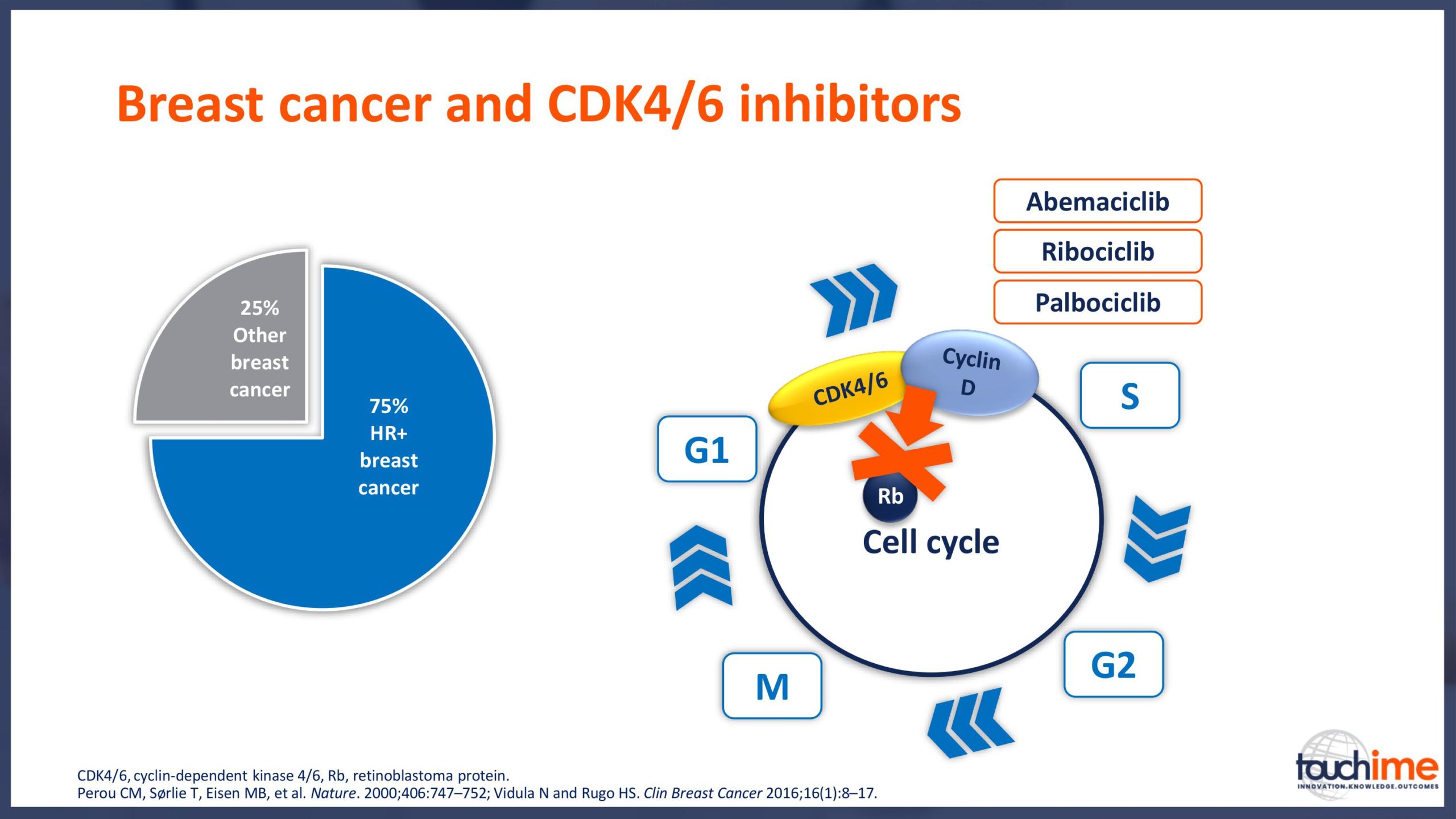
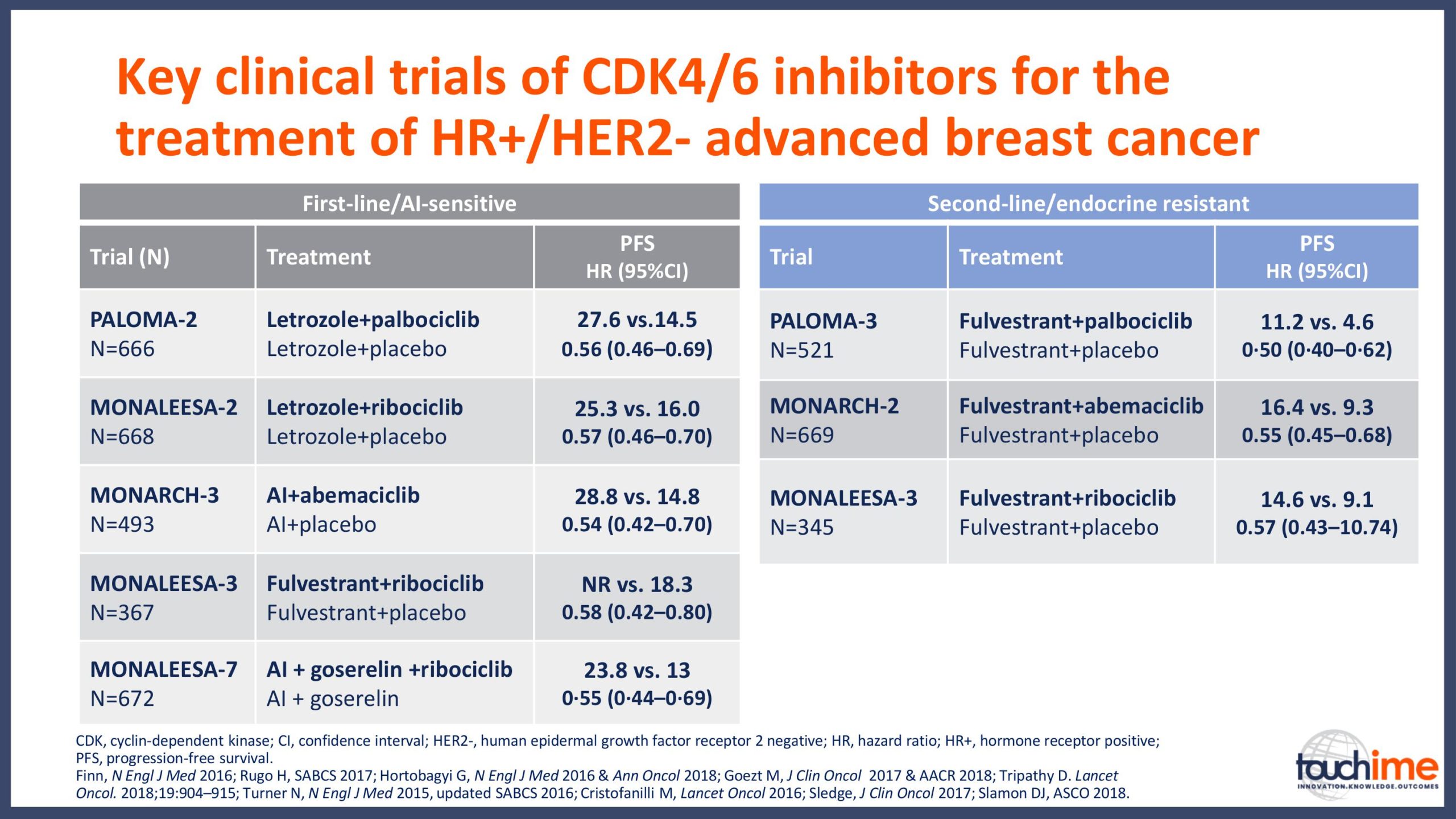
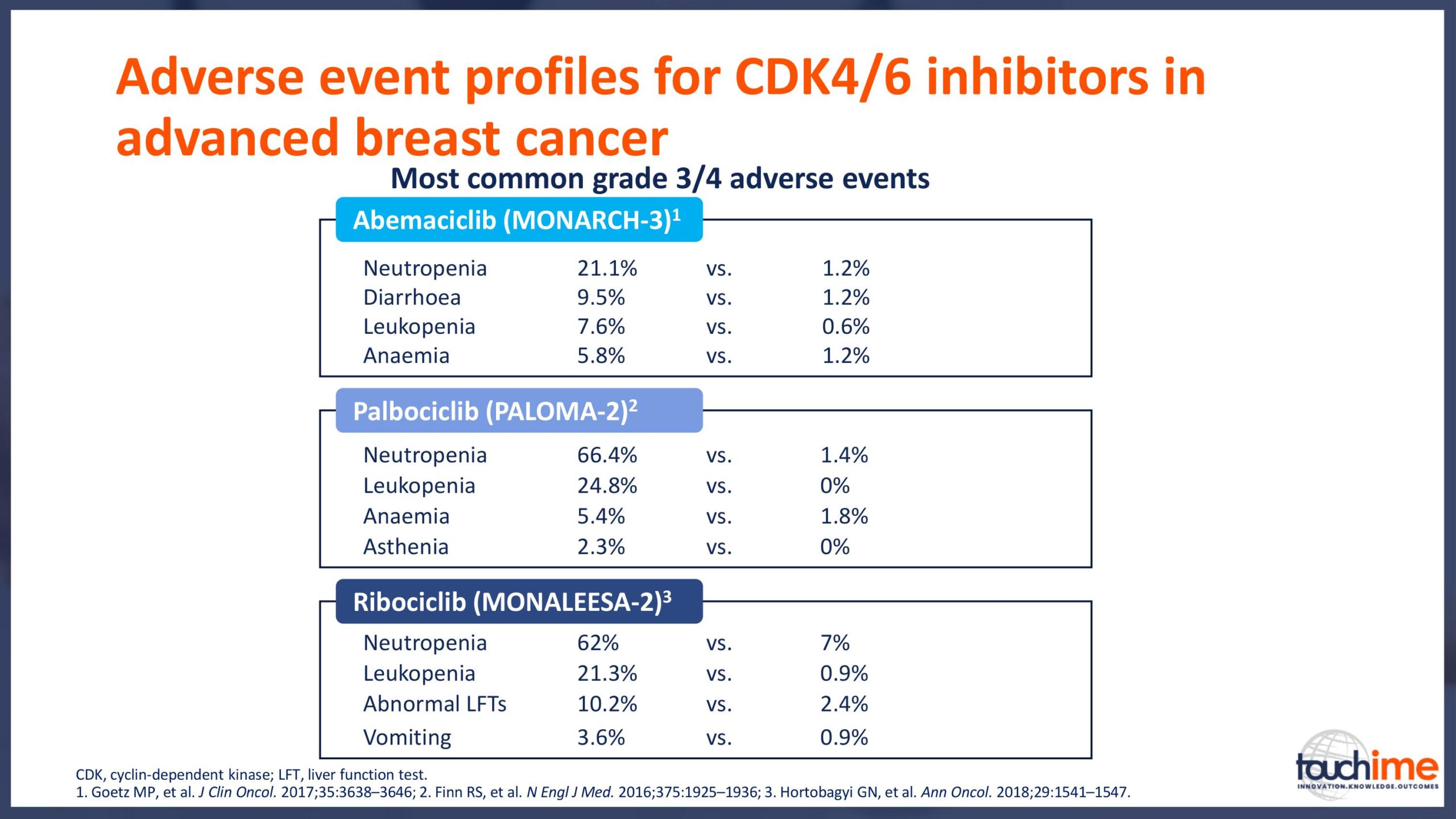
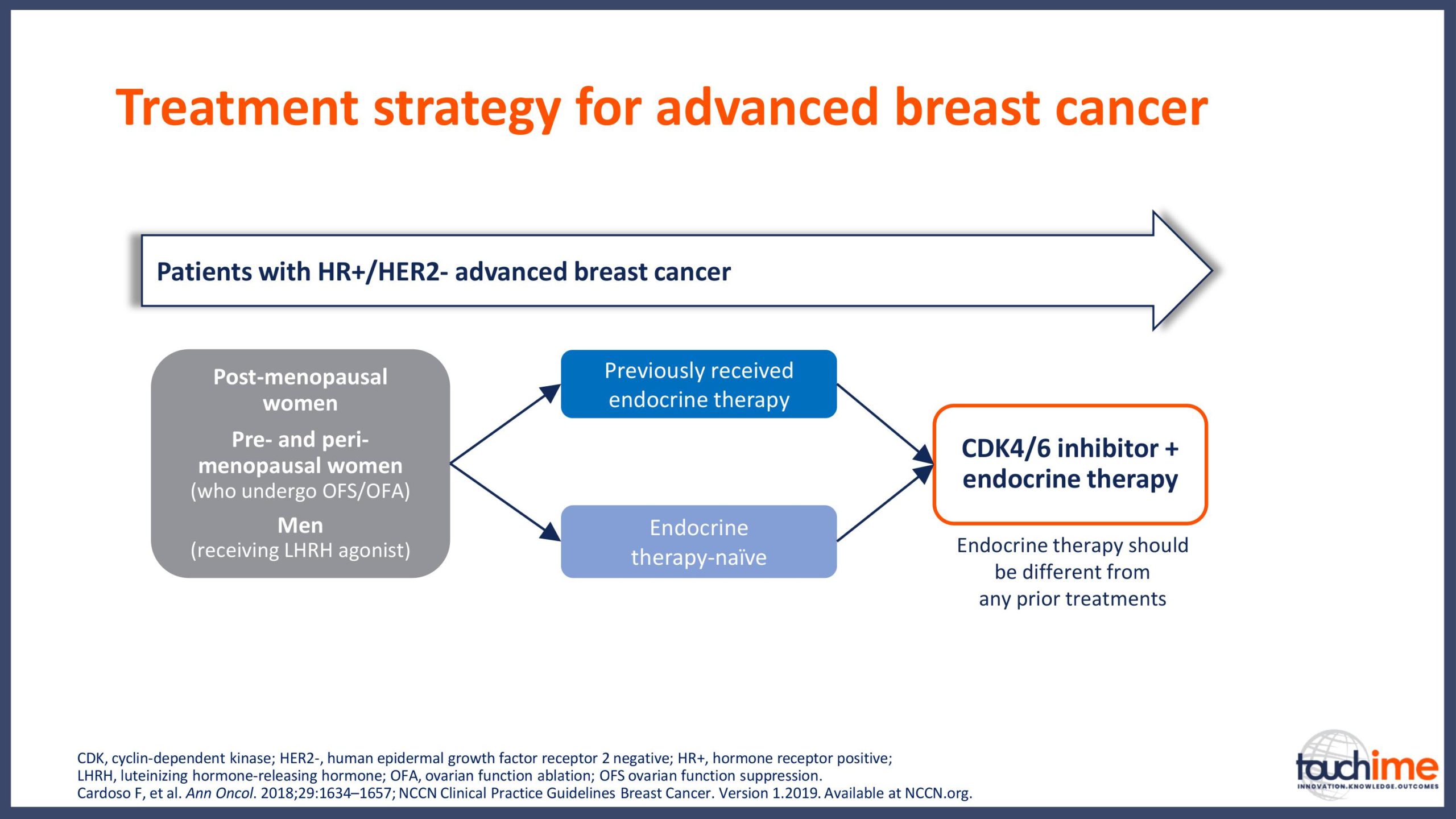
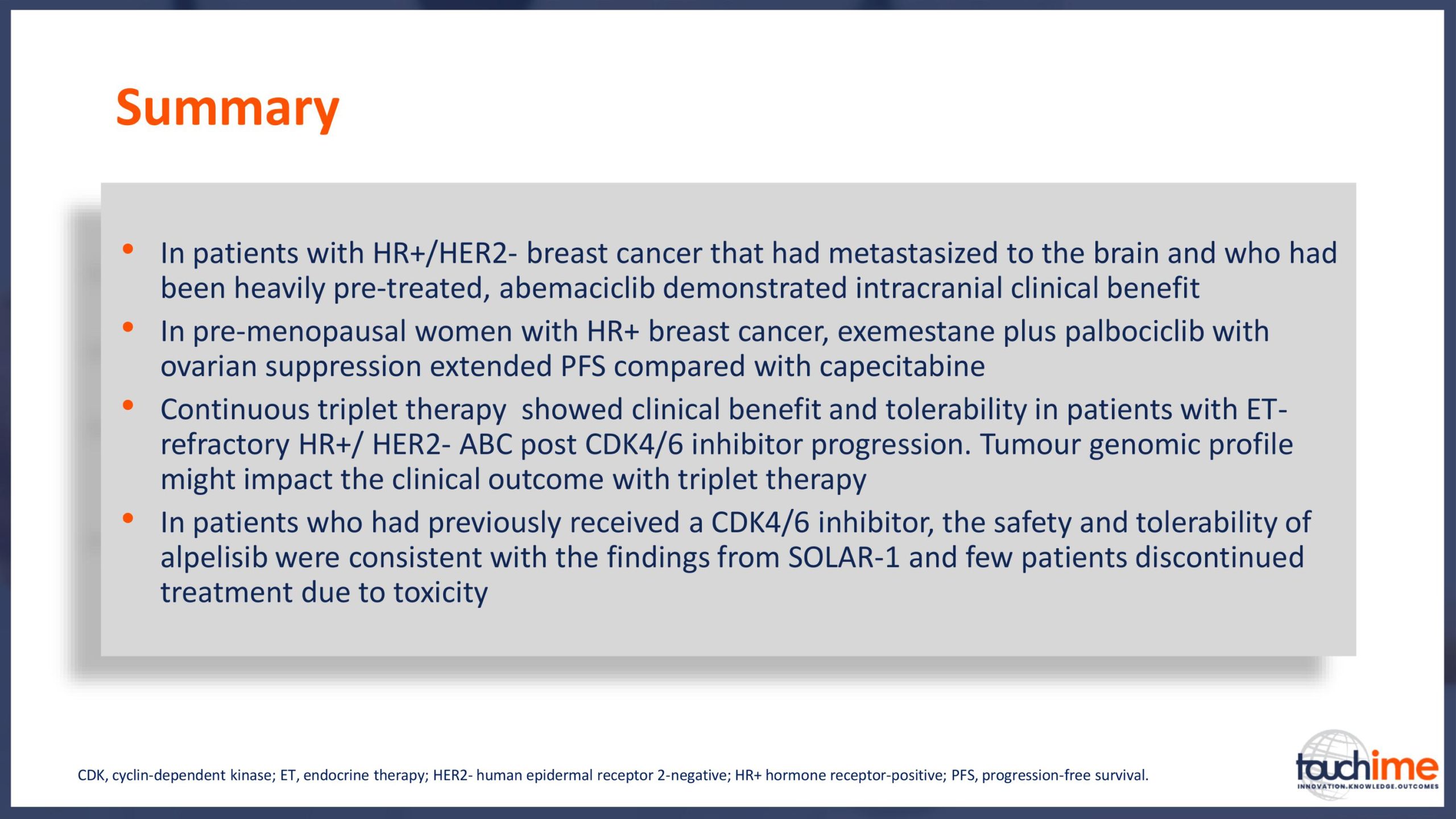
Current treatment landscape for CDK4/6 inhibitors
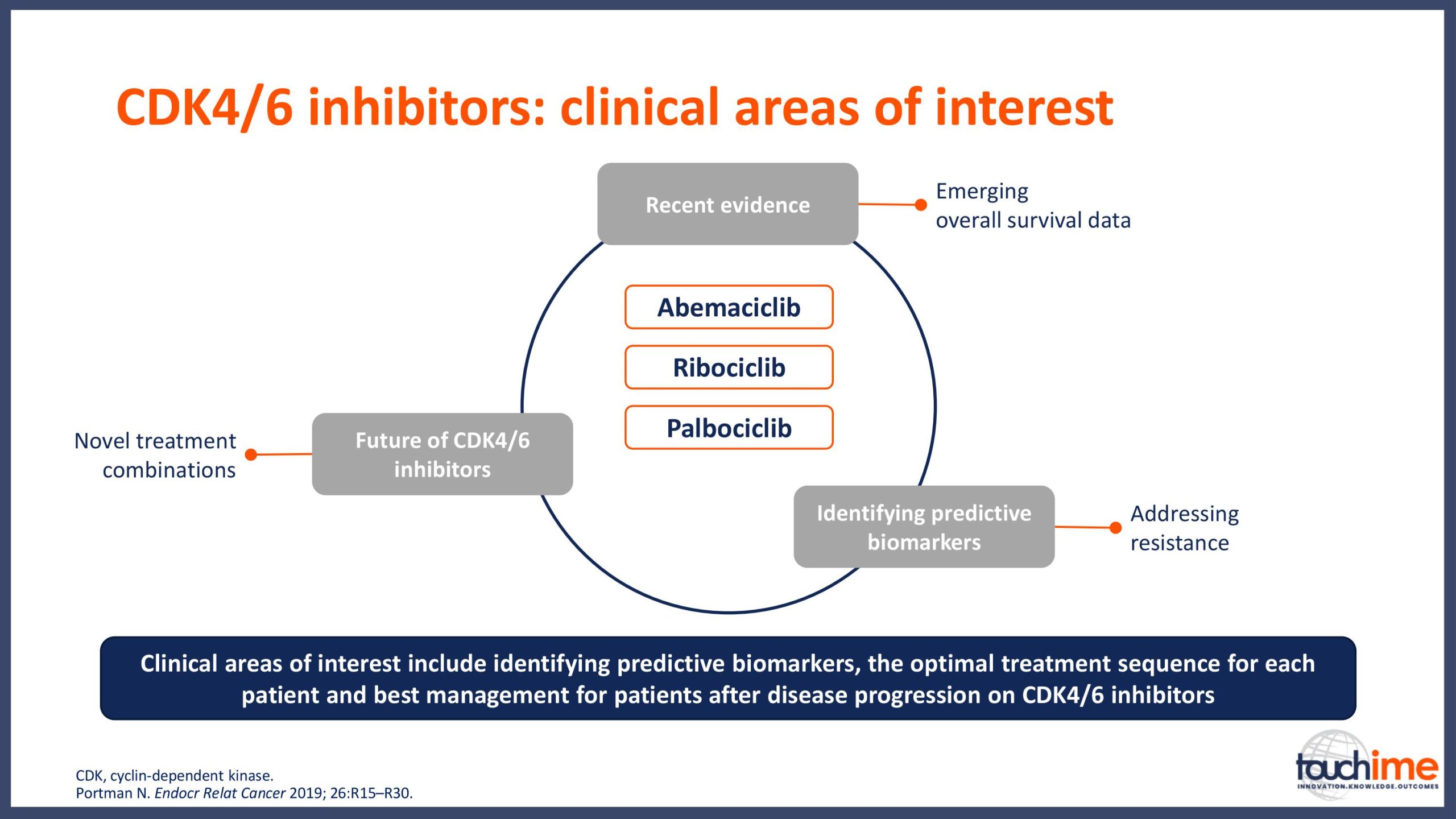
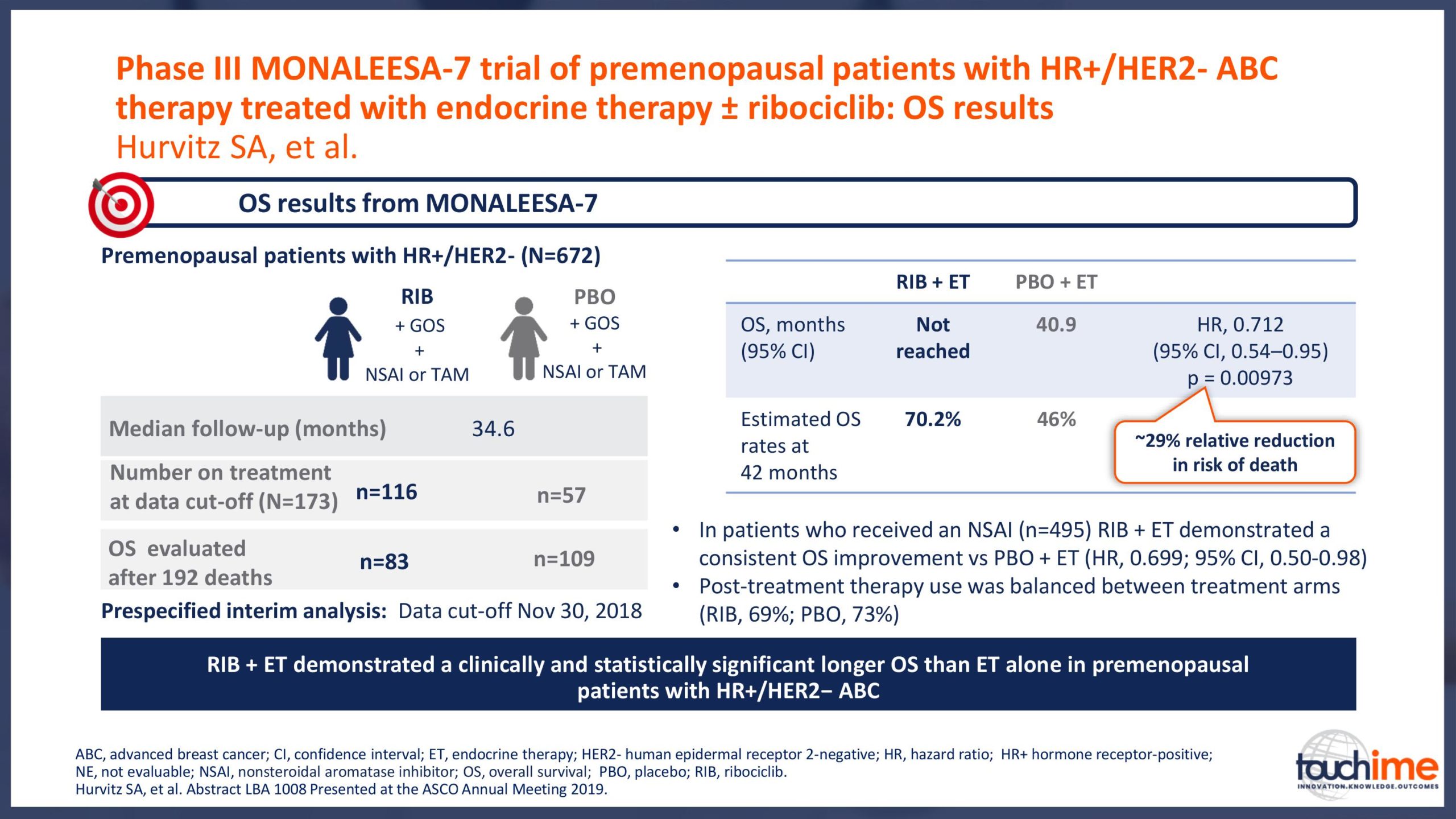
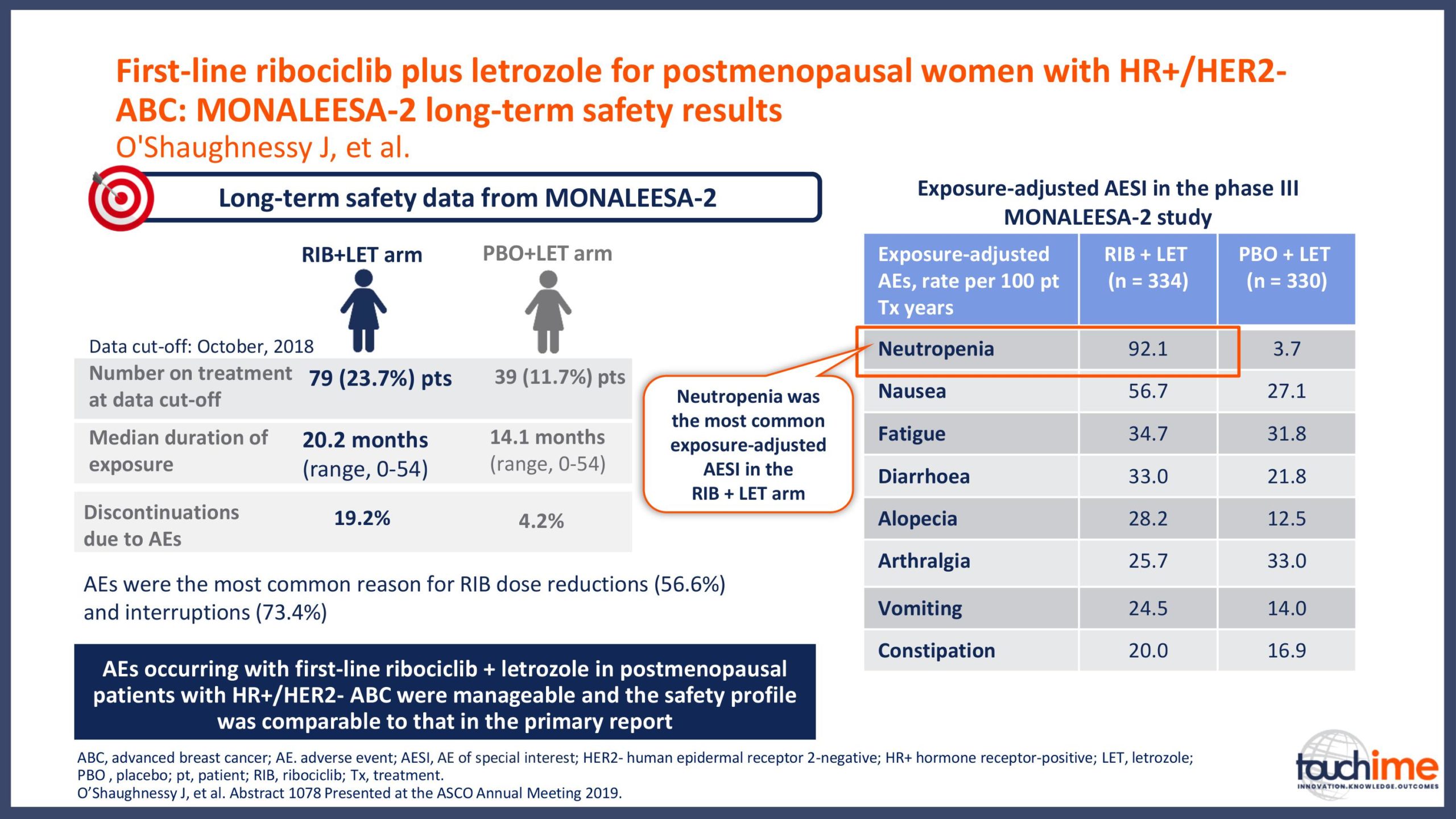
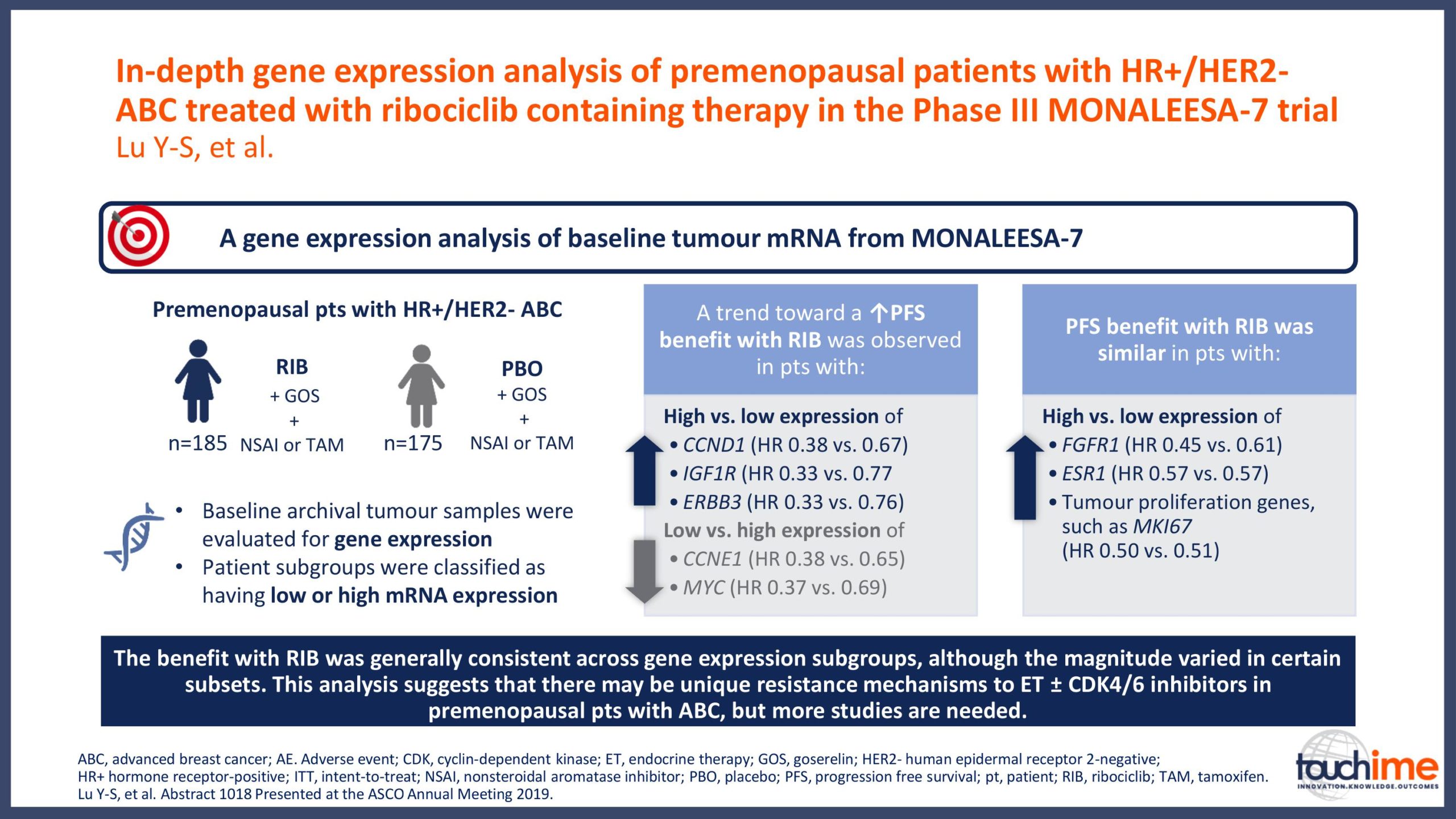
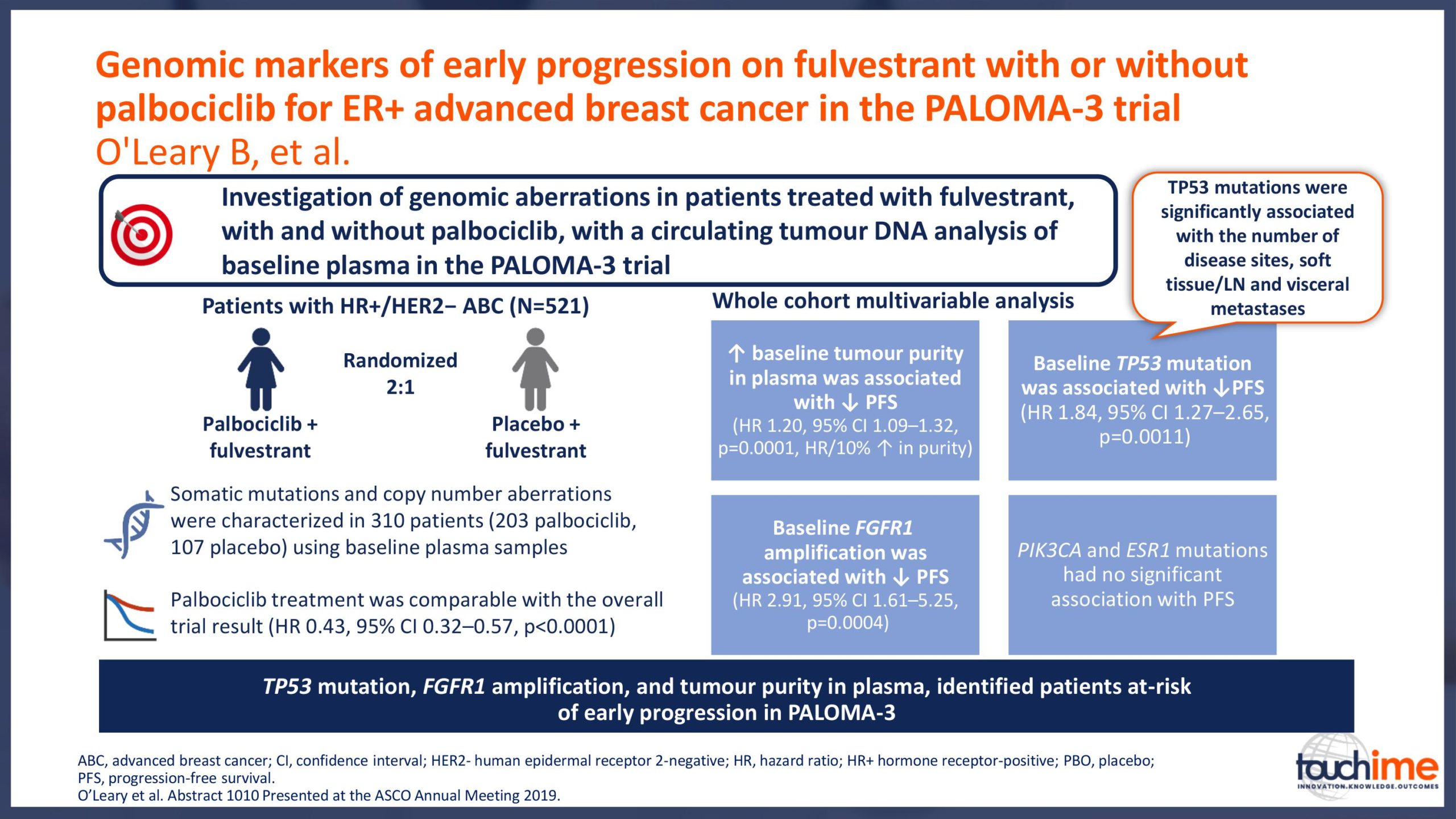
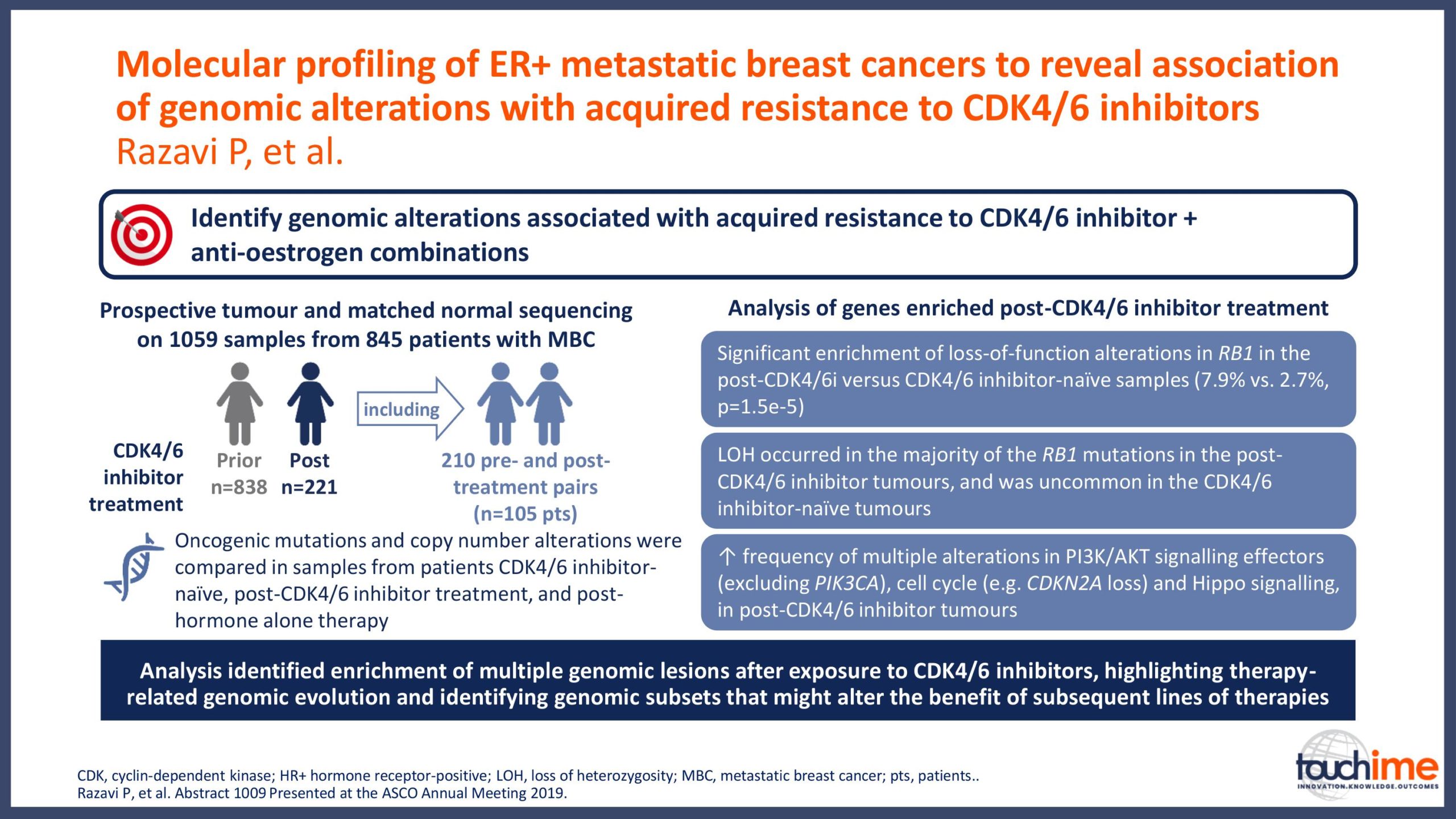
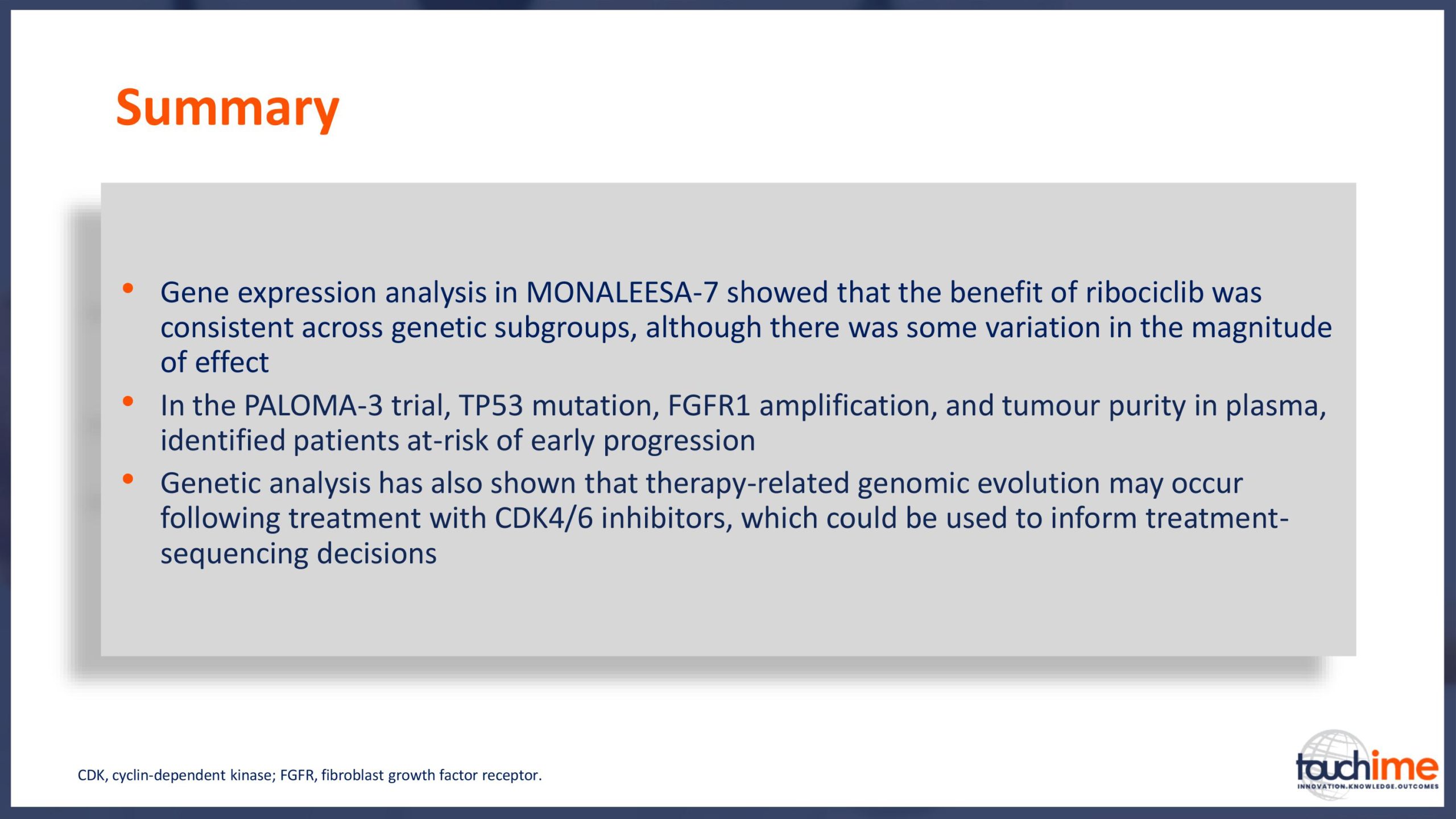
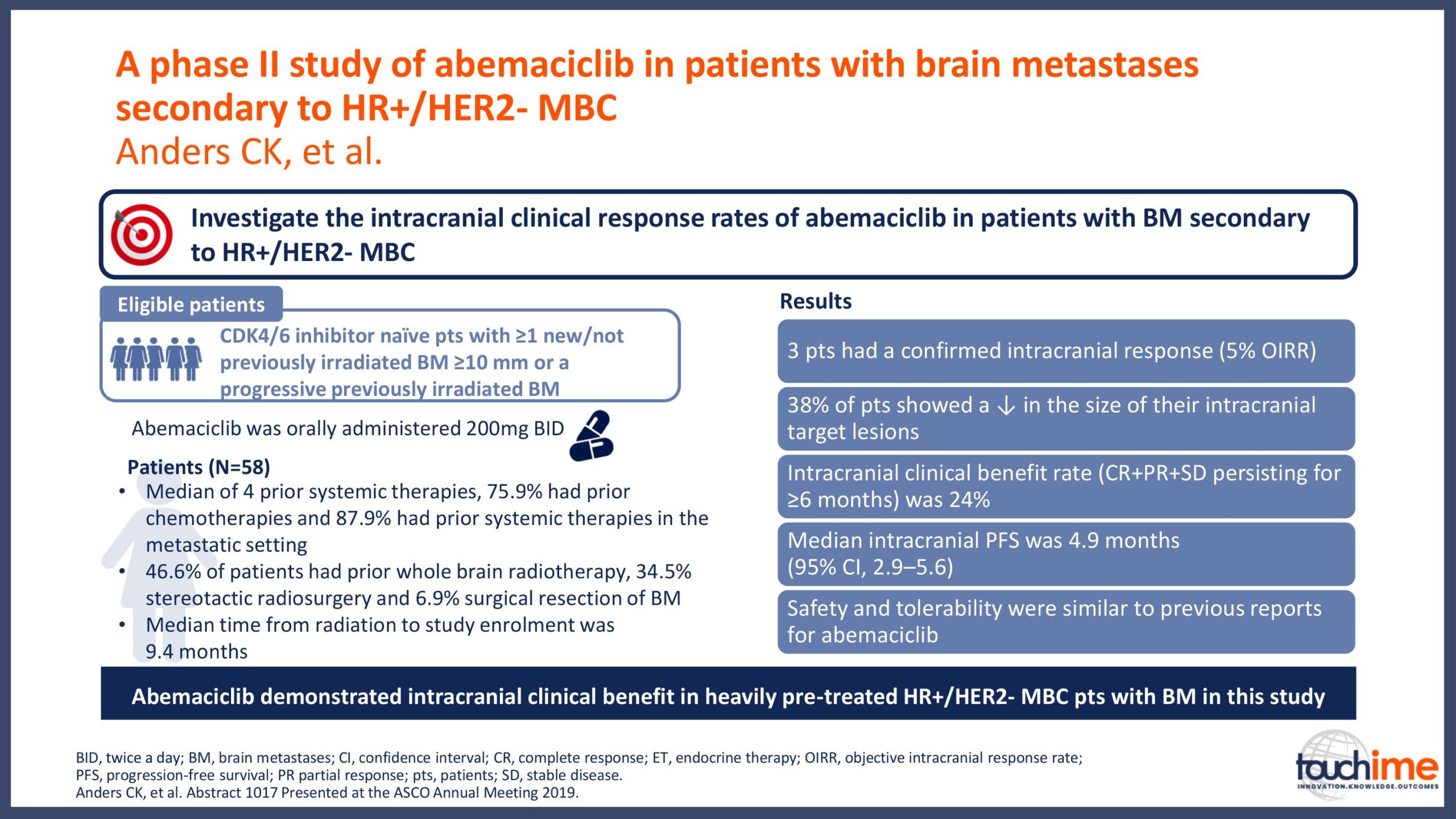
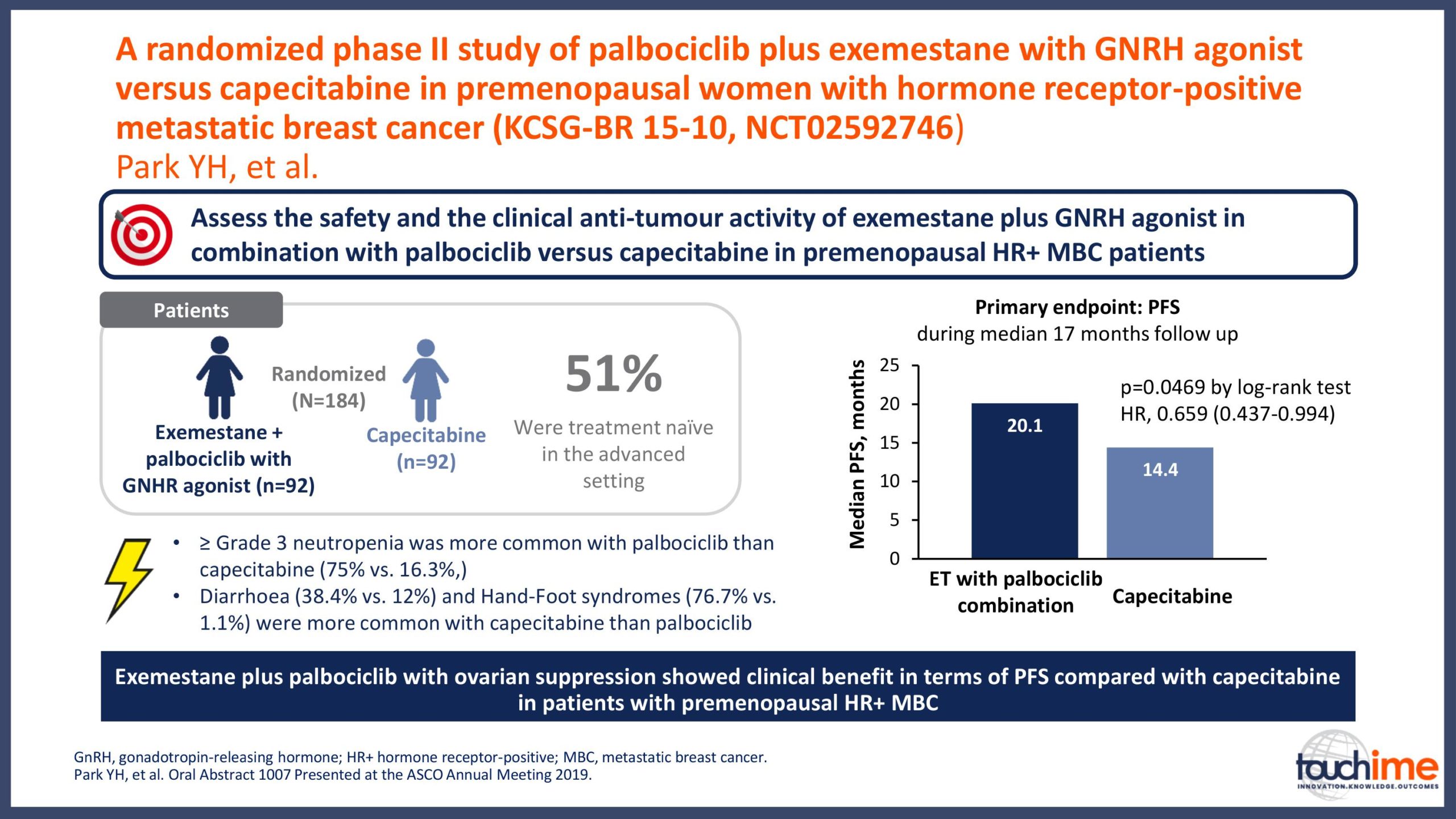
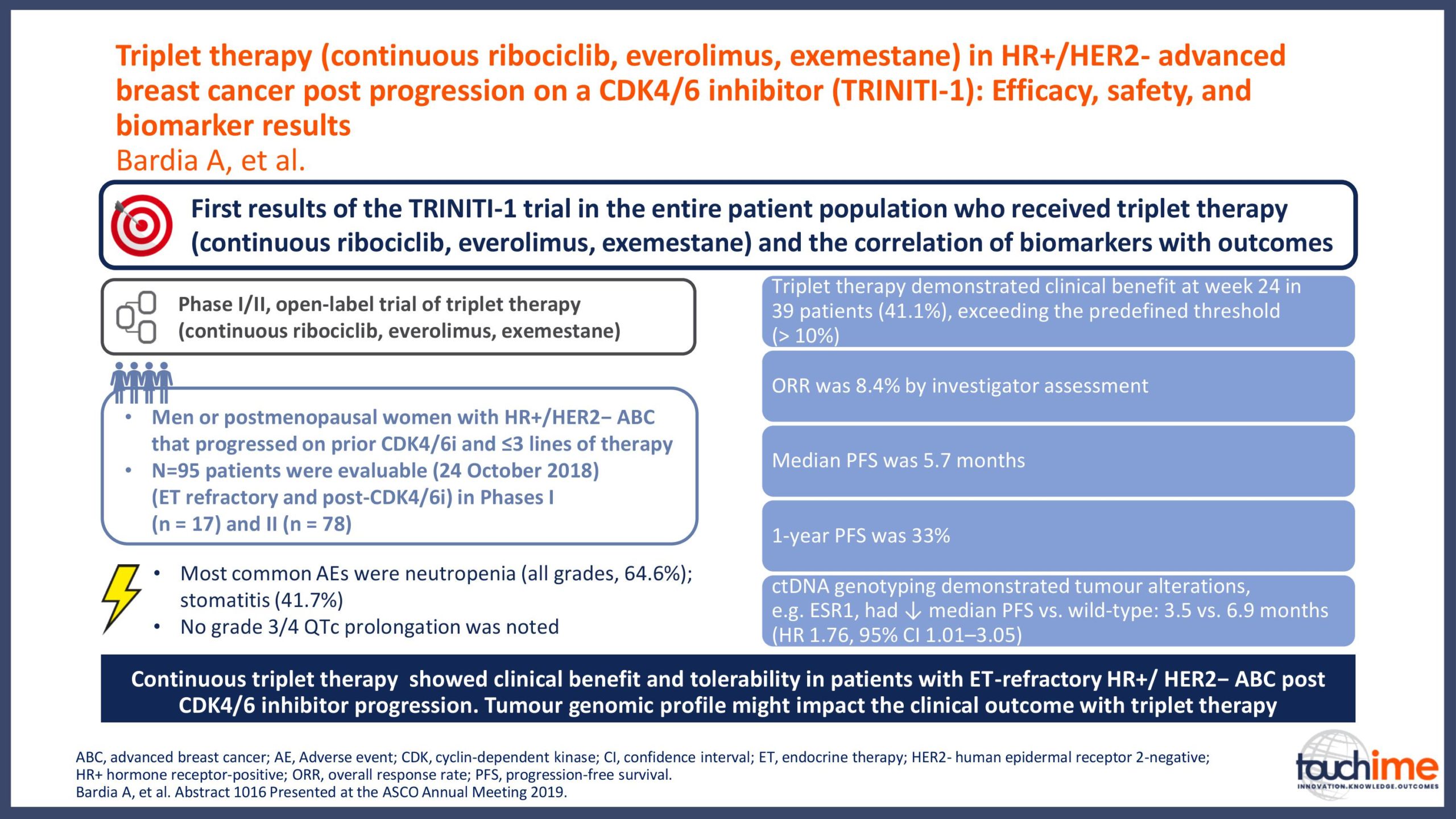
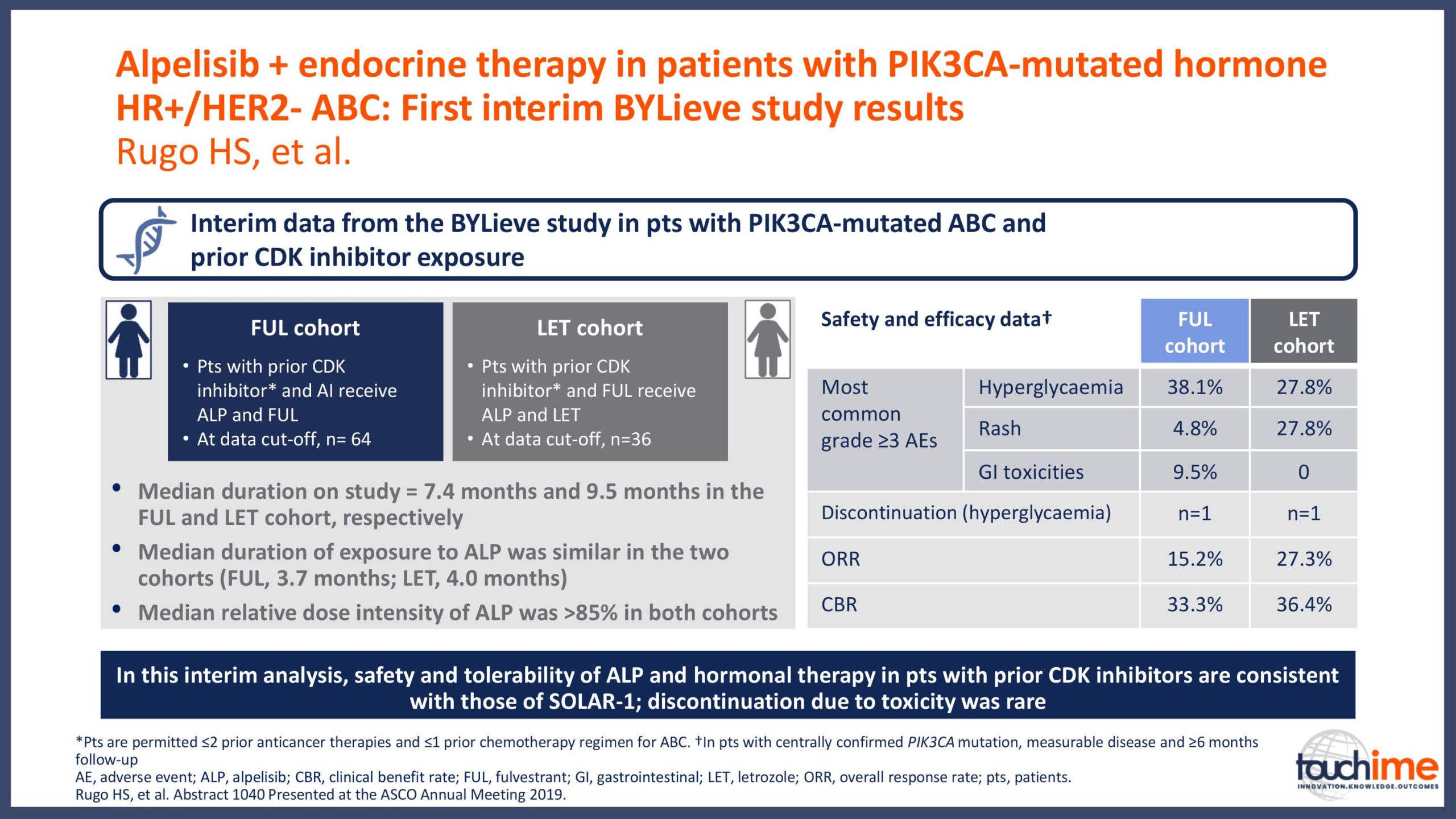
ASCO Annual Meeting 2019 - Recent evidence for CDK4/6 inhibitors
ASCO Annual Meeting 2019 - Identifying predictive biomarkers for CDK4/6 inhibitors: where are we now?
ASCO Annual Meeting 2019 - Next directions for CDK4/6 inhibitors
Overview
After watching this touchCONGRESS, you should be able to:
- Describe the role of CDK4/6 inhibitors in the context of the current and evolving treatment landscape for patients with HR+/HER2- advanced breast cancer
- Evaluate the importance of selecting the optimal treatment based on the individual patient, and the challenges around subsequent sequencing of therapy
- Summarize the importance of managing the safety profiles of CDK4/6 inhibitor therapy, and recognize the significance of the multidisciplinary team in optimizing patient outcomes and maintaining on-treatment benefits
Dr Joyce A. O’Shaughnessy, M.D. specializes in medical oncology with board certification in both internal medicine as well as medical oncology. She is a Diplomate of the American Board of Internal Medicine with subspecialty certification in medical oncology.
Dr O’Shaughnessy focuses her practice and clinical research on breast cancer treatment. She is Director of Breast Cancer Research at the Baylor-Sammons Cancer Center and Chair of Breast Cancer Research for The US Oncology Network.
Dr O’Shaughnessy received her M.D. from Yale University Medical School. Her internship and residency in internal medicine were completed at Massachusetts General Hospital in 1985. She concluded a fellowship in medical oncology at the National Cancer Institute in 1987 and was a Senior Investigator there until 1995.
Dr O’Shaughnessy’s memberships include the American Association for Cancer Research, the American Society of Clinical Oncology, the American College of Physicians and Women in Cancer Research. She is Associate Editor of Clinical Breast Cancer and Founder of the School of Breast Oncology.
Dr O’Shaughnessy has received the following Honours and Awards:
Giants of Cancer Care – Community Outreach/Education Award; Baylor University Medical Center – Staff Chief’s Award; Holy Cross College – Sanctae Crucis Award (Distinguished Alumni Award); Public Health Service Special Service – Development of the Consensus Statement on Cancer Drug Approval Endpoints Award; and the Yale Medical School – Francis Parker Award (Faculty Choice for Most Promising Clinician).
Her research interests include genotype-phenotype correlations for high risk breast cancers and also immunotherapy for triple negative breast cancer.
Dr Joyce A. O’Shaughnessy discloses: Honoraria as a speaker or consultant from AbbVie Inc., Agendia, Amgen Biotechnology, AstraZeneca, Bristol-Myers Squibb, Celgene Corporation, Eisai, Genentech, Genomic Health, GRAIL, Immunomedics, Heron Therapeutics, Ipsen Biopharmaceuticals, Jounce Therapeutics, Lilly,
Merck, Myriad, Novartis, Odonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Seattle Genetics and Syndax Pharmaceuticals.
Dr Véronique Diéras, Senior Medical Oncologist Head, Breast Cancer Group, Department of Medical Oncology, Centre Eugène Marquis, Rennes, France provides her expert insight into key data presented at ASCO 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Sherko Kümmel, Clinical Director and Chairman of the Interdisciplinary Breast Unit and Lead of the Breast Cancer Research Programme at Kliniken Essen-Mitte, Germany, provides his expert insight into key data presented at the ASCO Annual Meeting 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Dr Patrick Neven, oncologist at the Universitair Ziekenhuis Leuven provides his expert insight into key data presented at the ASCO Annual Meeting 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Joohyuk Sohn, Professor at the Yonsei Cancer Center, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea, provides his expert insight into key data presented at the ASCO Annual Meeting 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Dr. Joyce A. O’Shaughnessy, Director of Breast Cancer Research at the Baylor-Sammons Cancer Center, Texas and Chair of Breast Cancer Research for The US Oncology Network, provides her expert insight into key data presented at the ASCO Annual Meeting 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof, Michelino De Laurentiis, Chair, Department of Breast and Thoracic Oncology, National Cancer Institute ‘Fondazione Pascale’, Naples, Italy, provides his expert insight into key data presented at the ASCO Annual Meeting 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Please Select A Video:
Overview & Learning Objectives
Overview
Stay up-to-date with the latest developments in CDK4/6 inhibition for advanced breast cancer with our expert summary from the American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, United States, 31 May–4 June 2019.
The information in this activity is intended for oncologists, nurses, and other healthcare professionals involved in the treatment of patients with breast cancer.
Learning Objectives
Watch Joyce A. O'Shaughnessy reviewing the most important emerging data presented at the ASCO Annual Meeting in Chicago, United States, 31 May–4 June 2019 and discussing their potential impact for treating patients with HR+/HER2- advanced breast cancer, including:
- What does the treatment landscape look like for HR+/HER2- advanced breast cancer, now and in the future? Focus on the impact of CDK4/6 inhibitors
- What are the latest findings from the ASCO Annual Meeting 2019 for CDK4/6 inhibitors in patients with HR+/HER2- advanced breast cancer? Focus on optimizing treatment sequencing
- What do the data from the ASCO Annual Meeting 2019 tell us about the safety of CDK4/6 inhibitors? Focus on maximizing patient quality of life

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights