touchCONGRESS HR+/HER2- Advanced breast cancer: what are the latest developments in CDK4/6 inhibition from the ESMO Breast Cancer Congress 2019?
Stay up-to-date with the latest developments in CDK4/6 inhibition for advanced breast cancer with our expert summary from the ESMO Breast Cancer Congress in Berlin, Germany, 2–4 May 2019.
Part 1: Watch internationally renowned expert Prof. Sibylle Loibl review key data from ESMO Breast 2019
Part 2: Choose from leading experts who discuss what the data findings mean for global and regional practice
Introduction
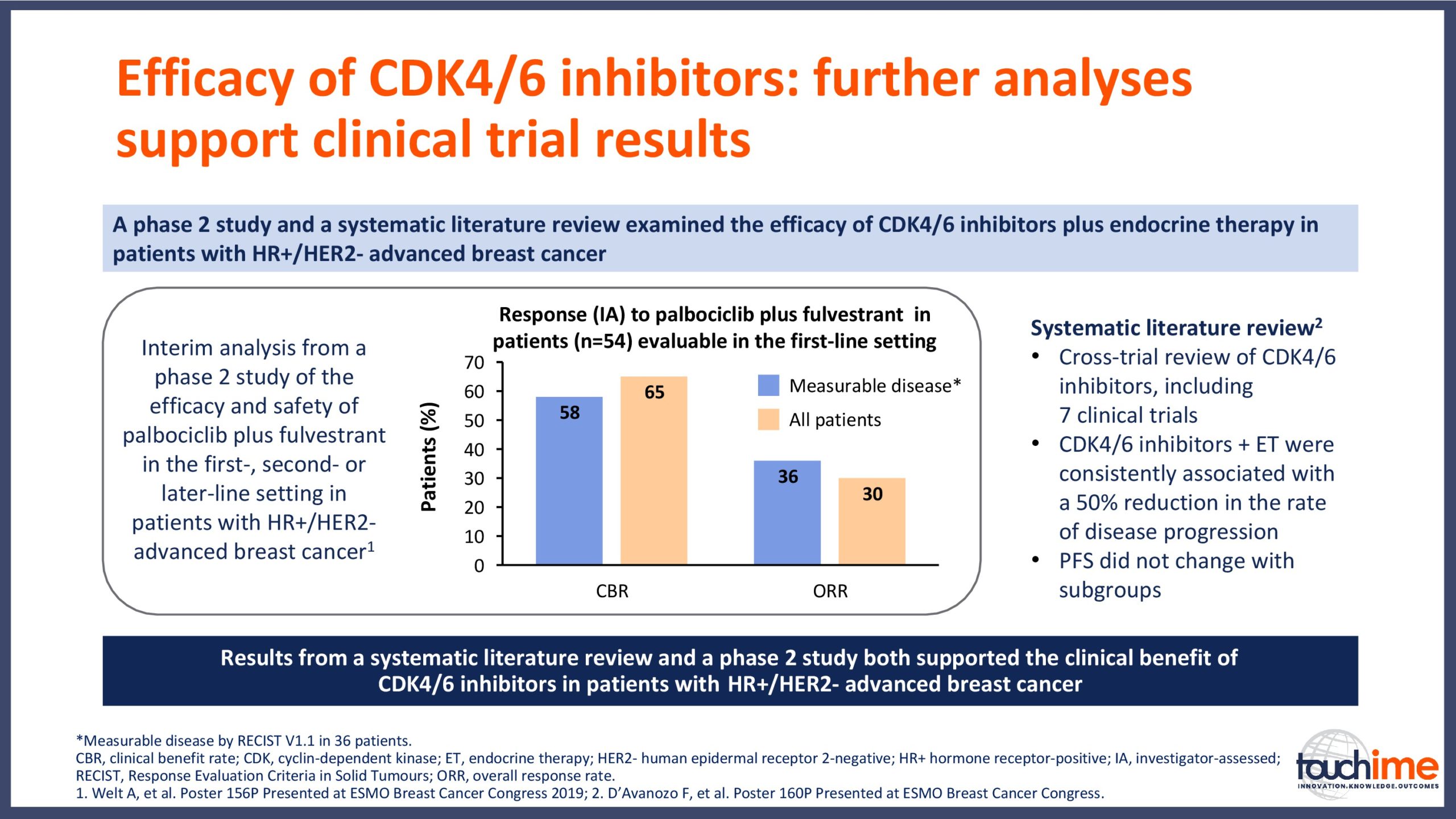
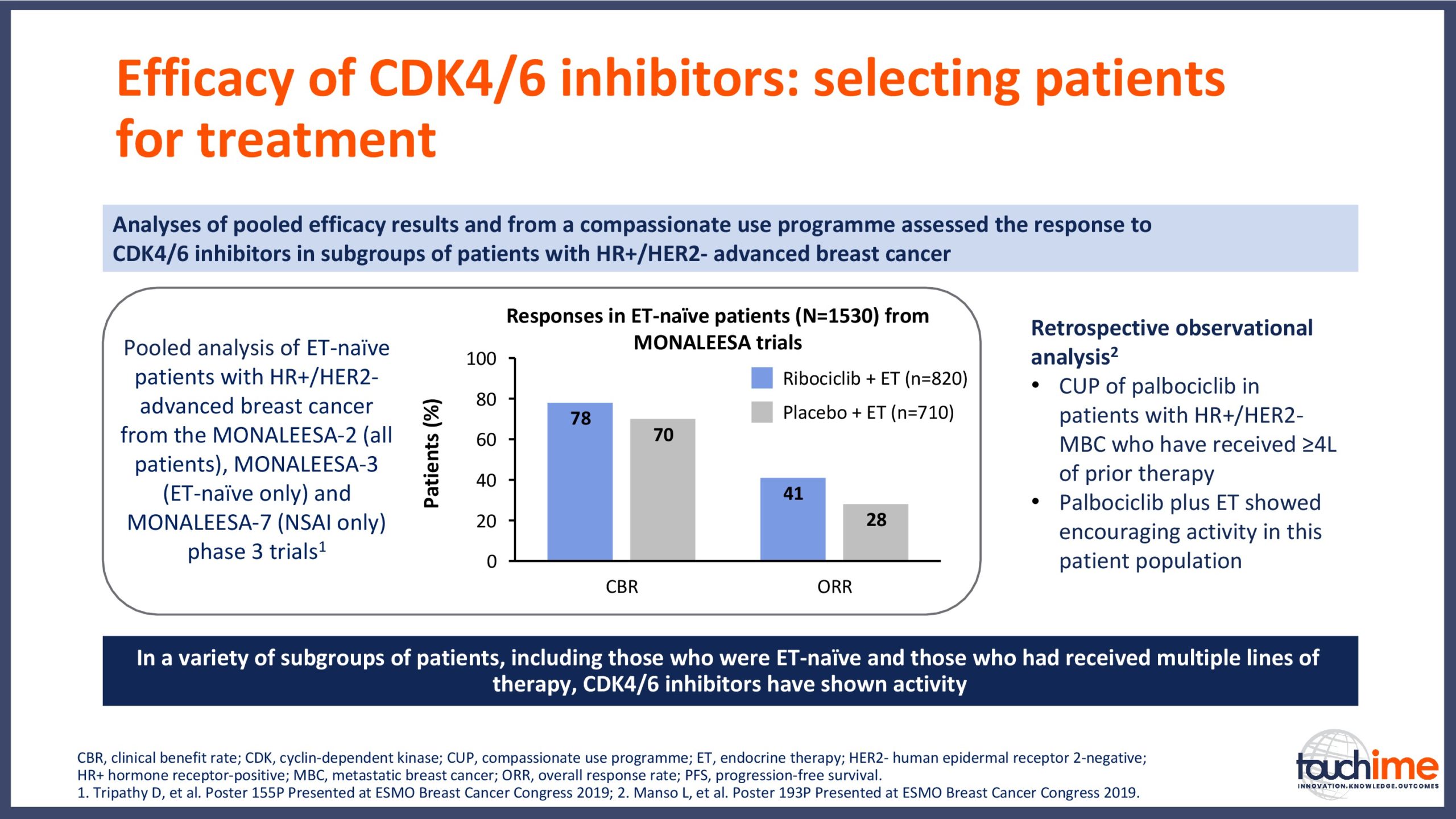
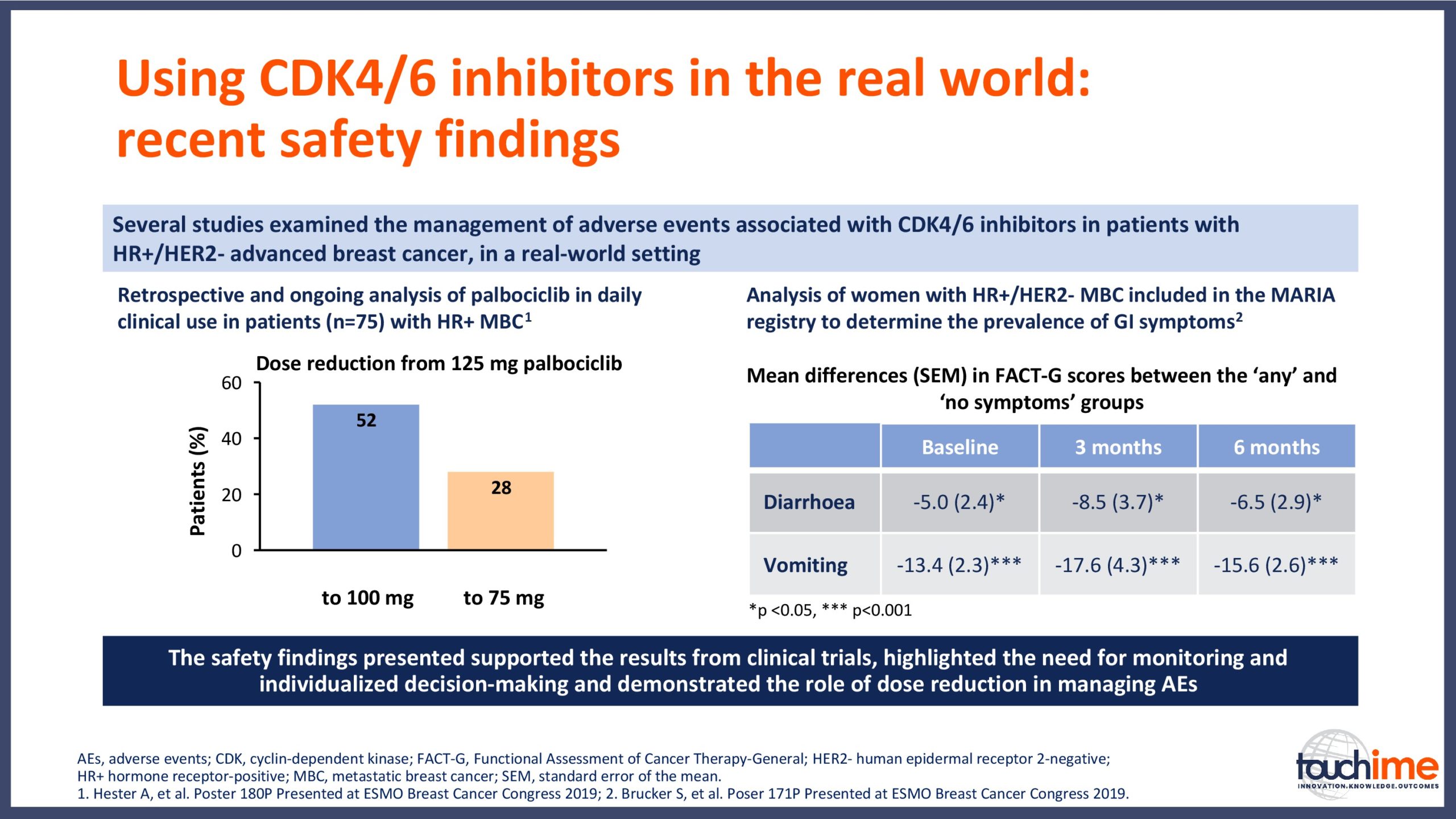
ESMO Breast Cancer Congress 2019 – Latest findings on the role of CDK4/6 inhibitors
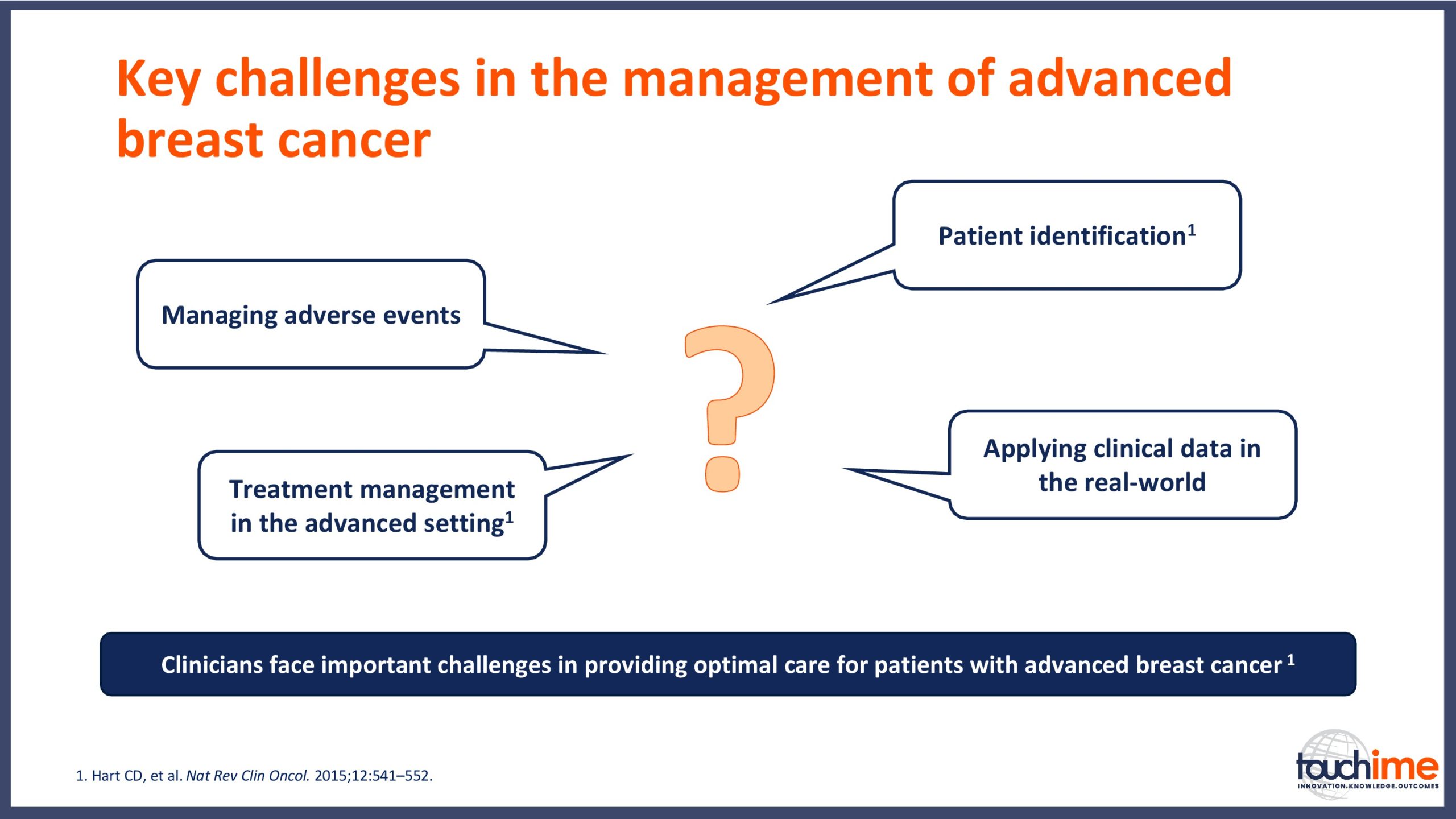
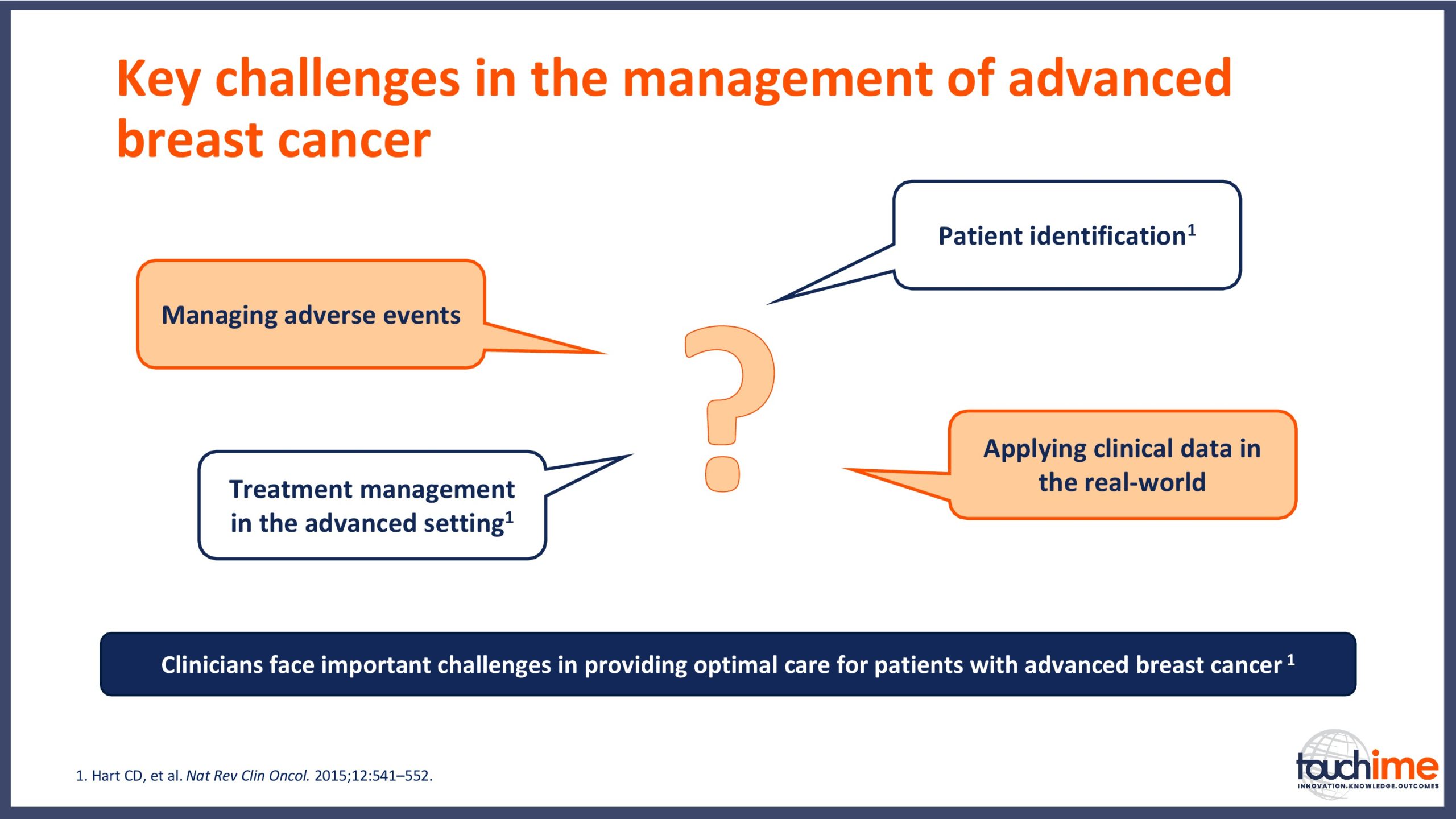
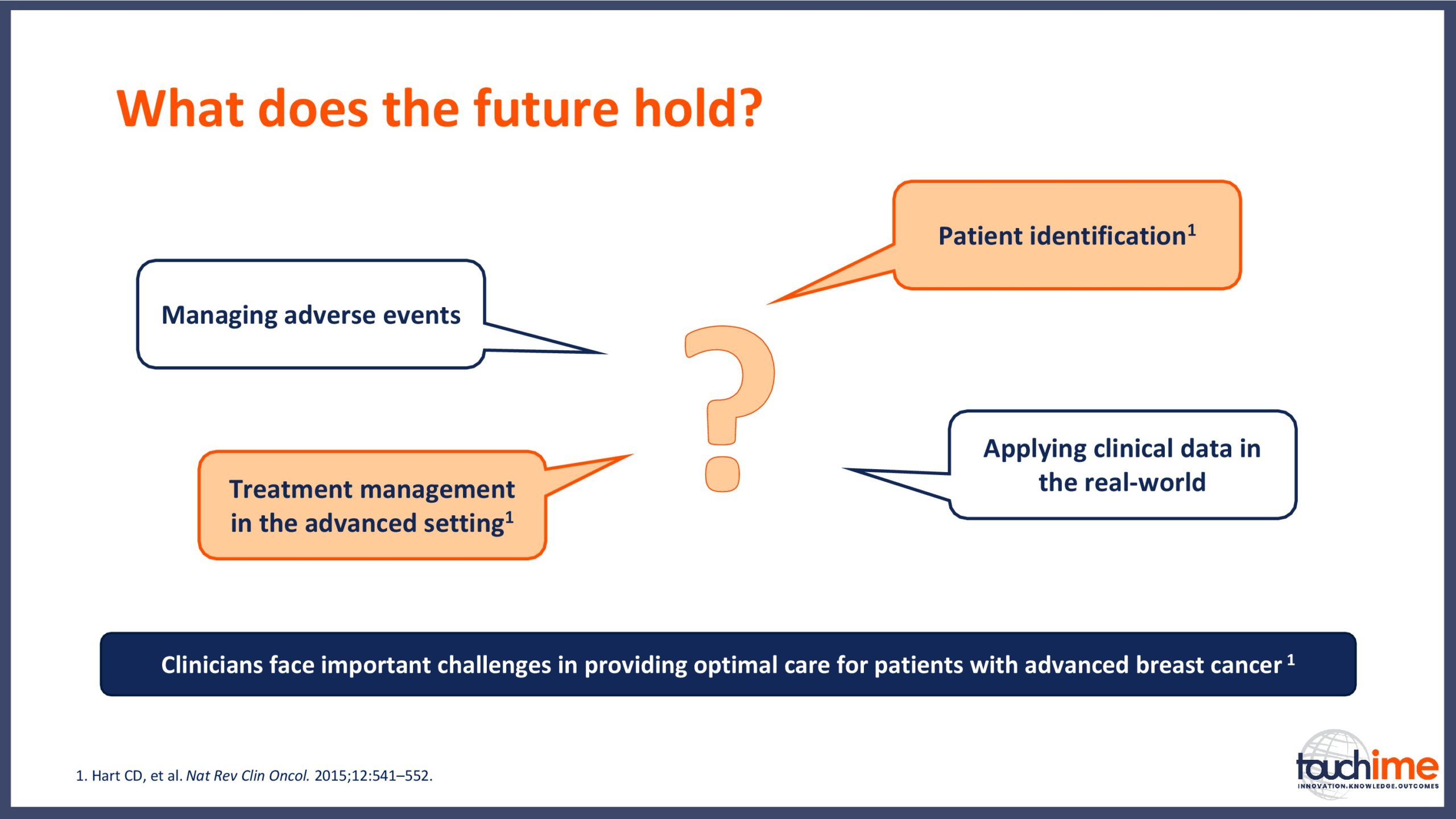
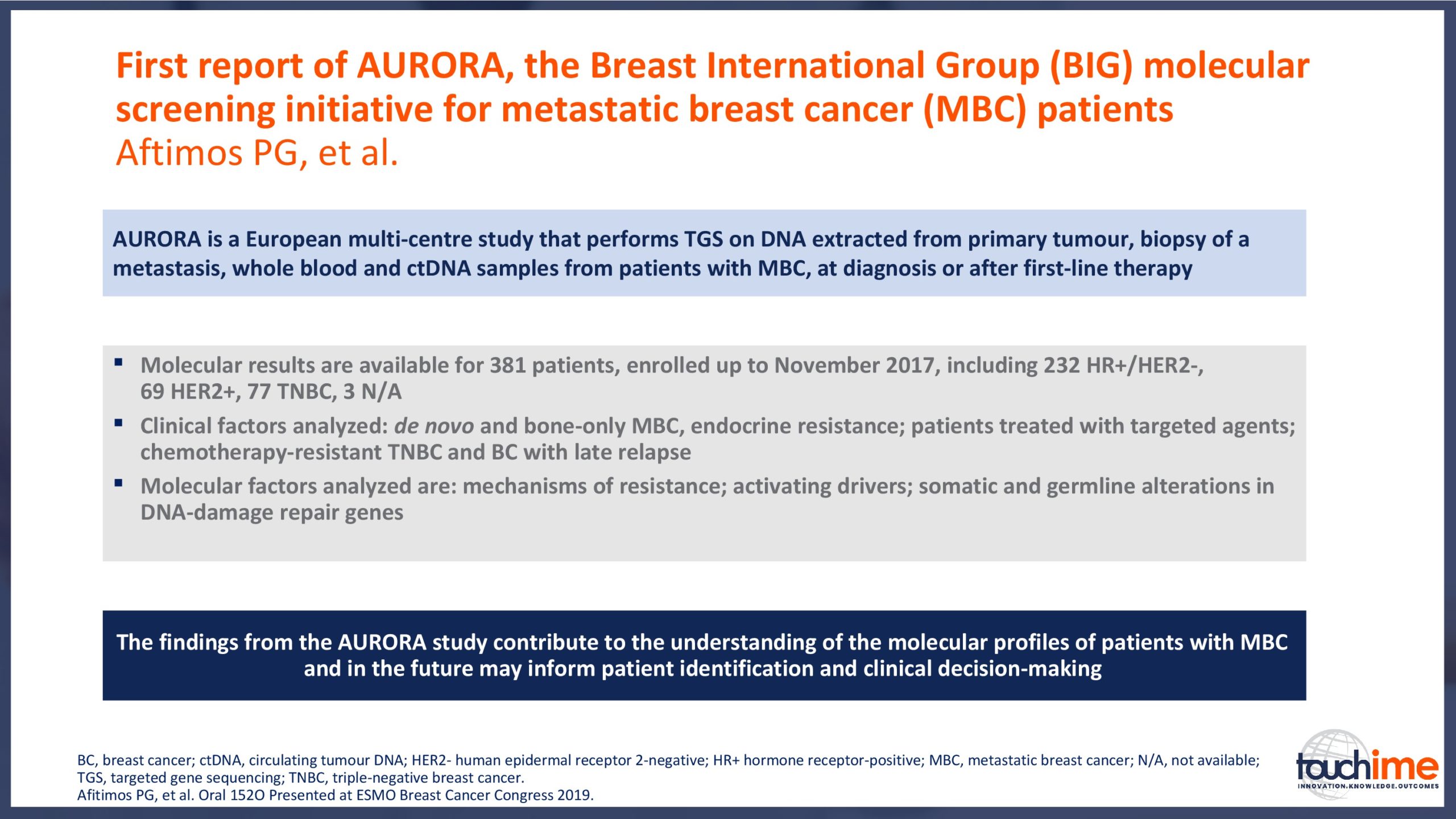
ESMO Breast Cancer Congress 2019 – What does the future hold for patient identification and selection?
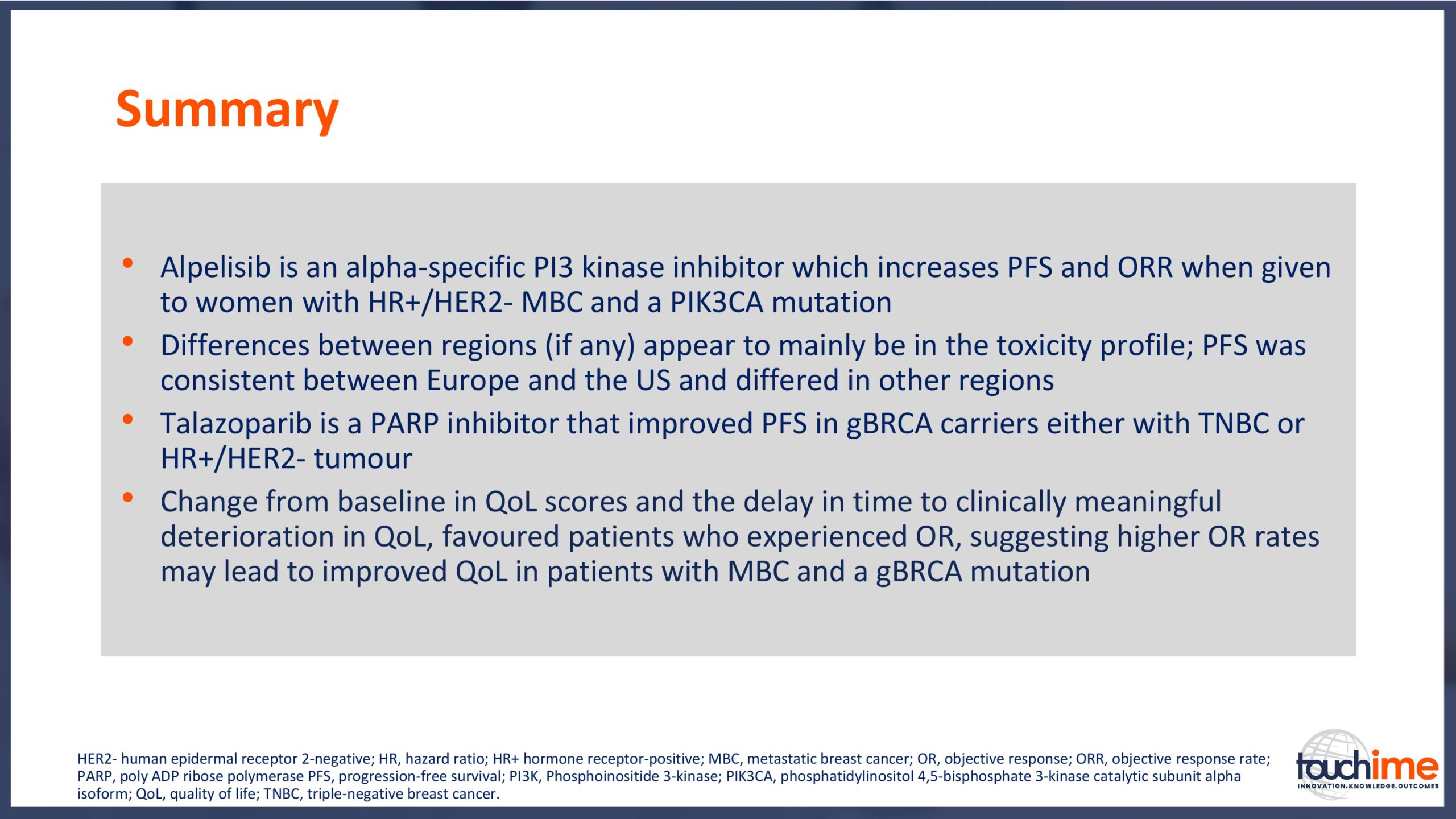
ESMO Breast Cancer Congress 2019 – What does the future hold for treatment management in the advanced setting?
Overview
Watch Prof. Sibylle Loibi reviewing the most important emerging data presented at the ESMO Breast Cancer Congress in Berlin, Germany, 2–4 May 2019 and discussing their potential impact for treating patients with HR+/HER2- advanced breast cancer, including:
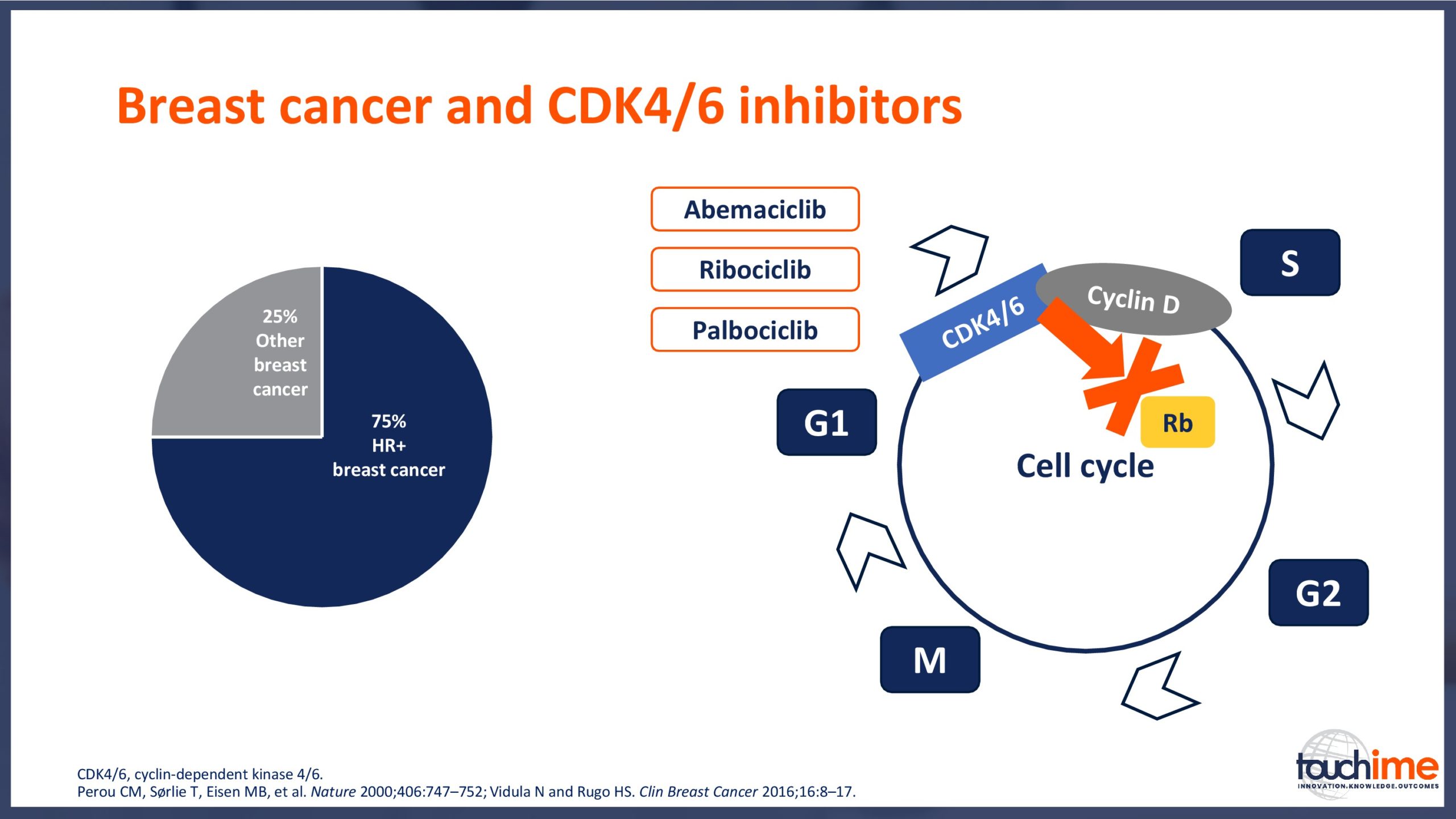
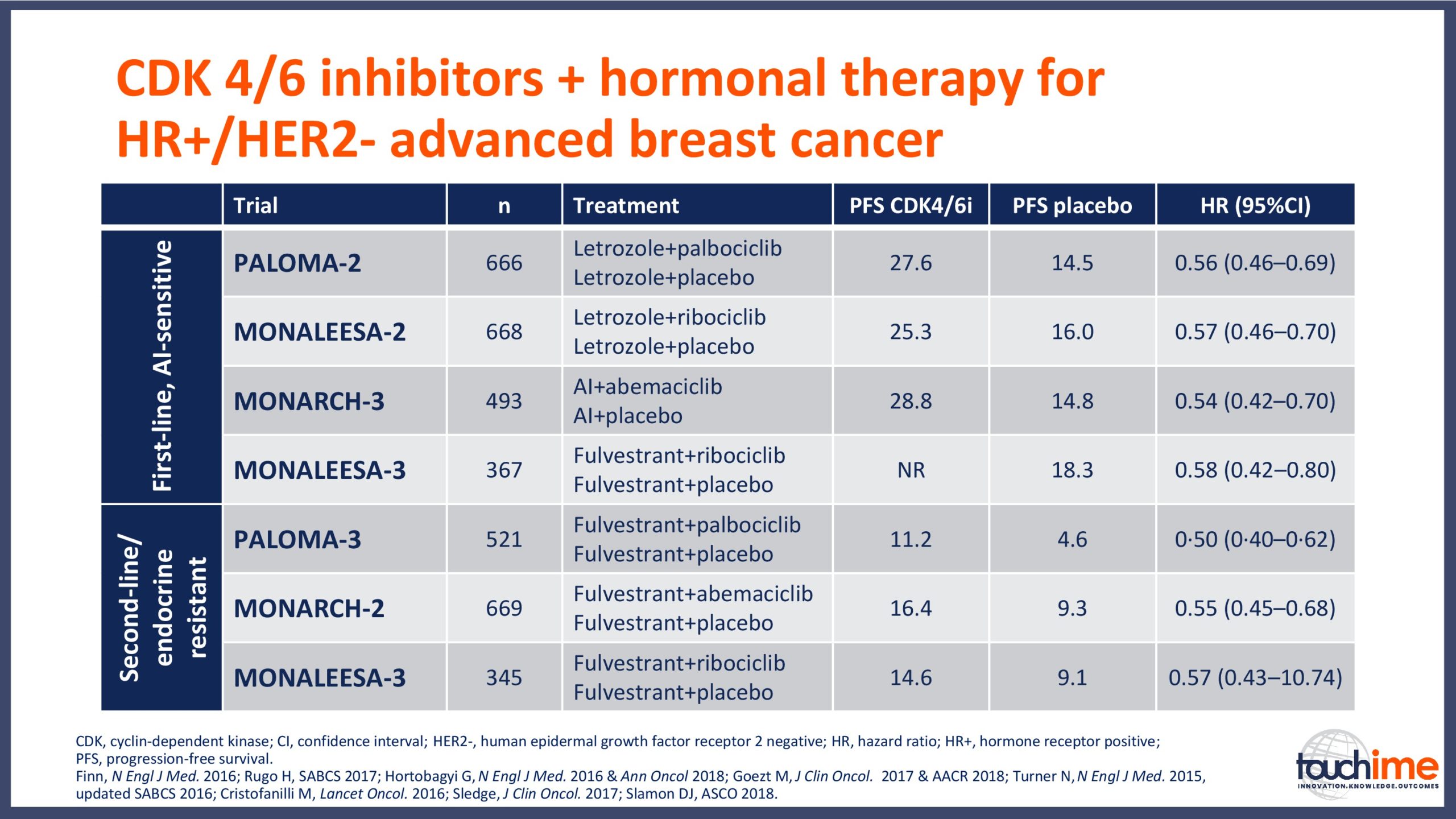
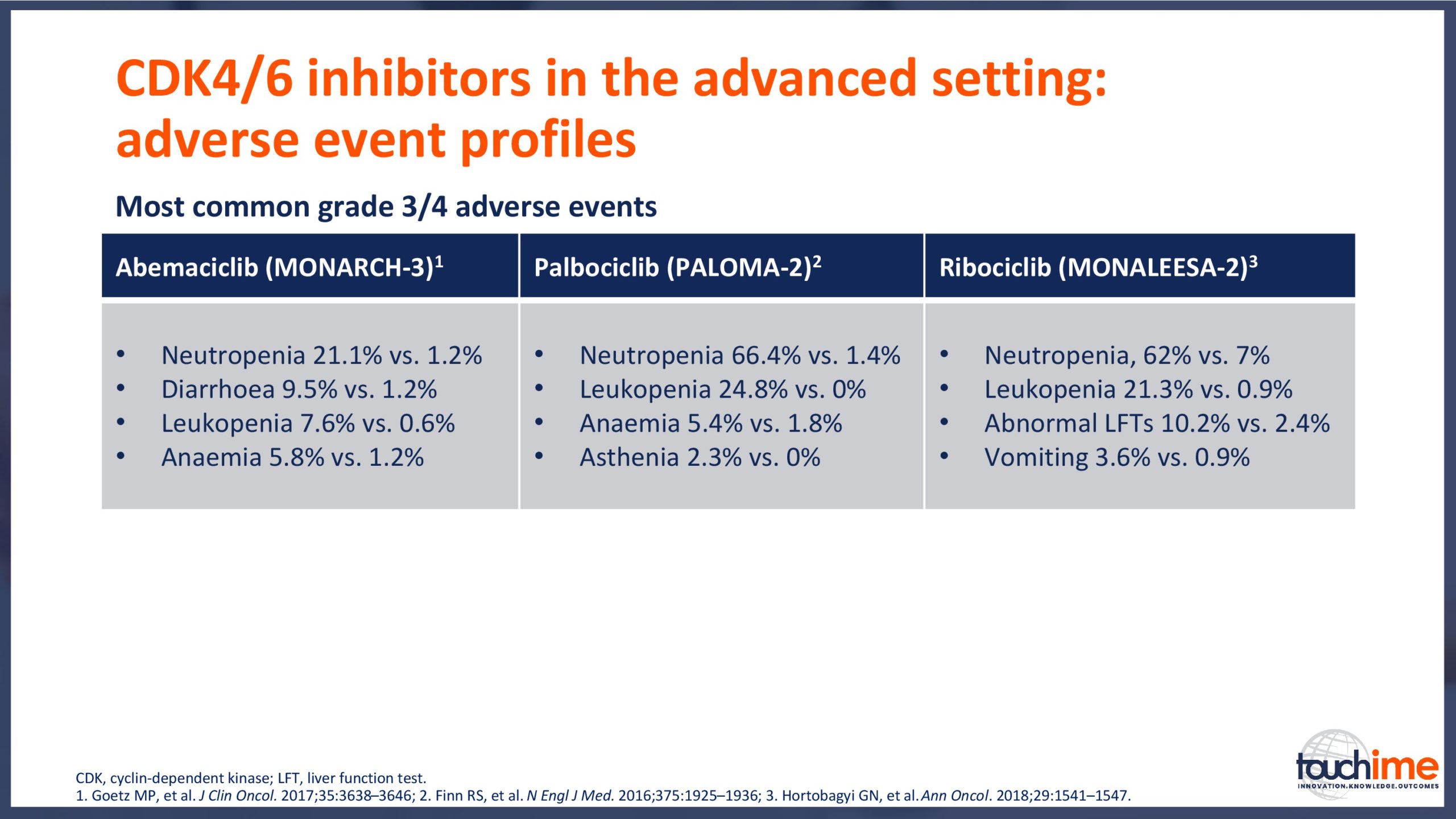
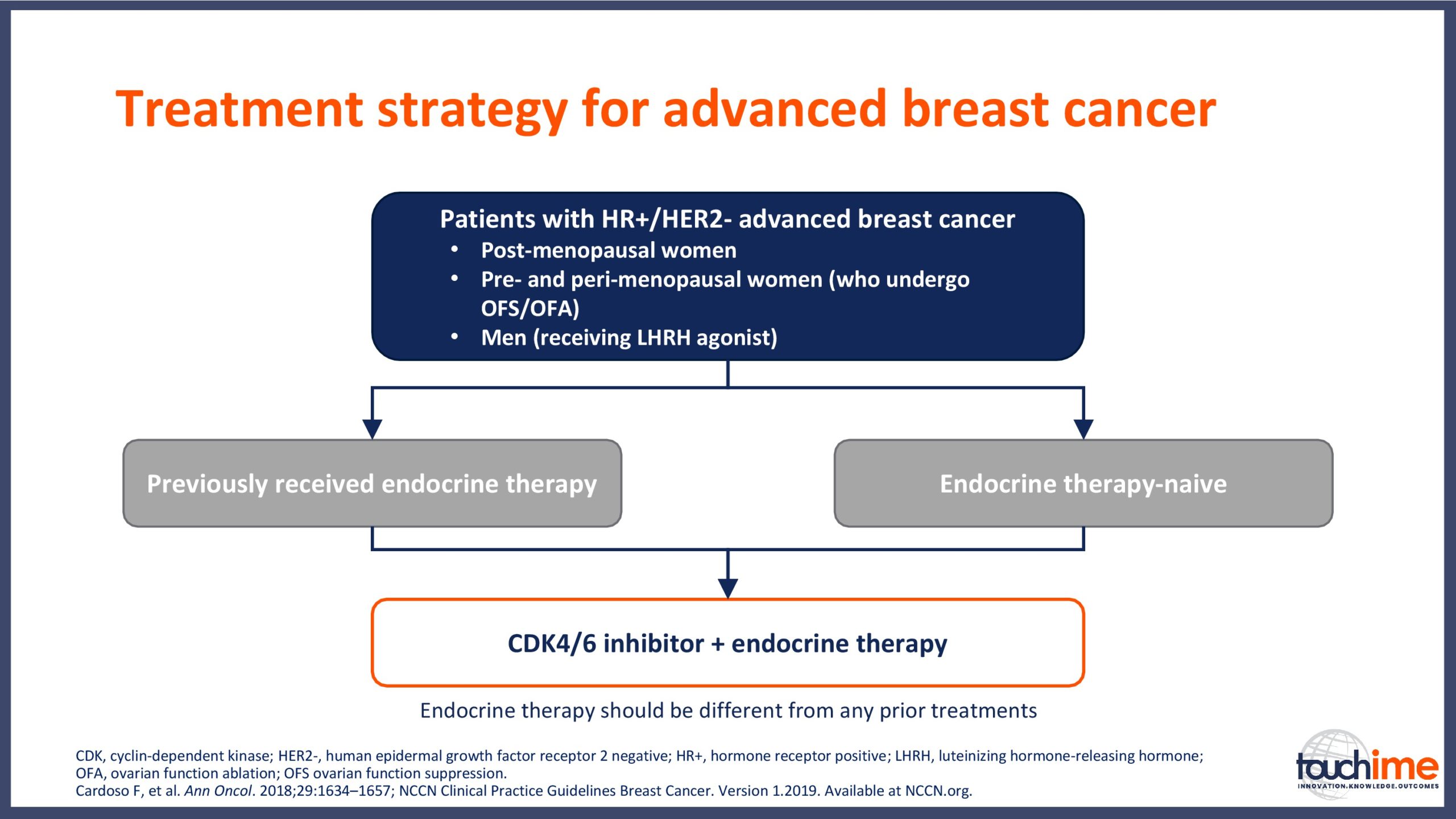
- What are the latest results for CDK4/6 inhibitors in patients with advanced HR+/HER2- breast cancer? Focus on optimal use and the management of adverse events
- How can treatment be personalized for patients with metastatic HR+/HER2- breast cancer? Focus on mutational analysis for patient selection
- How is the management of patients with advanced HR+/HER2- breast cancer evolving? Focus on assessing treatment response and managing outcomes
Prof. Sibylle Loibl, MD, PhD, is the Chief Executive Officer and Chair of the German Breast Group (GBG), one of the leading study groups worldwide. She devotes the majority of her time to clinical trial research at the GBG and at her clinical affiliations: the University of Frankfurt, and Oncology Bethanien in Frankfurt.
Prof. Loibl is an associate professor at the University of Frankfurt. She graduated from the University of Heidelberg and completed her fellowship and residency as a gynaecologist and obstetrician at the Universities of Heidelberg and Frankfurt.
Prof. Loibl is internationally renowned in the fields of neoadjuvant breast cancer, breast cancer during pregnancy, and breast cancer in young women. She developed the translational research division within the GBG and has led numerous translational projects as well as research projects funded by the EU Commission. She has led and participated in more than 100 national and international clinical trials in the field of breast cancer.
In 2014, she joined the Executive Board of the Breast International Group (BIG). As a medical expert, she serves on several international steering committees, translational research committees, and independent data monitoring committees.
Prof. Loibl has co-authored almost 300 MEDLINE-listed scientific papers, in addition to more than 200 original and peer-reviewed articles and 30 books or book chapters. She has also actively contributed to more than 200 national and international congresses. She is an active member of numerous national and international organizations such as the American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO).
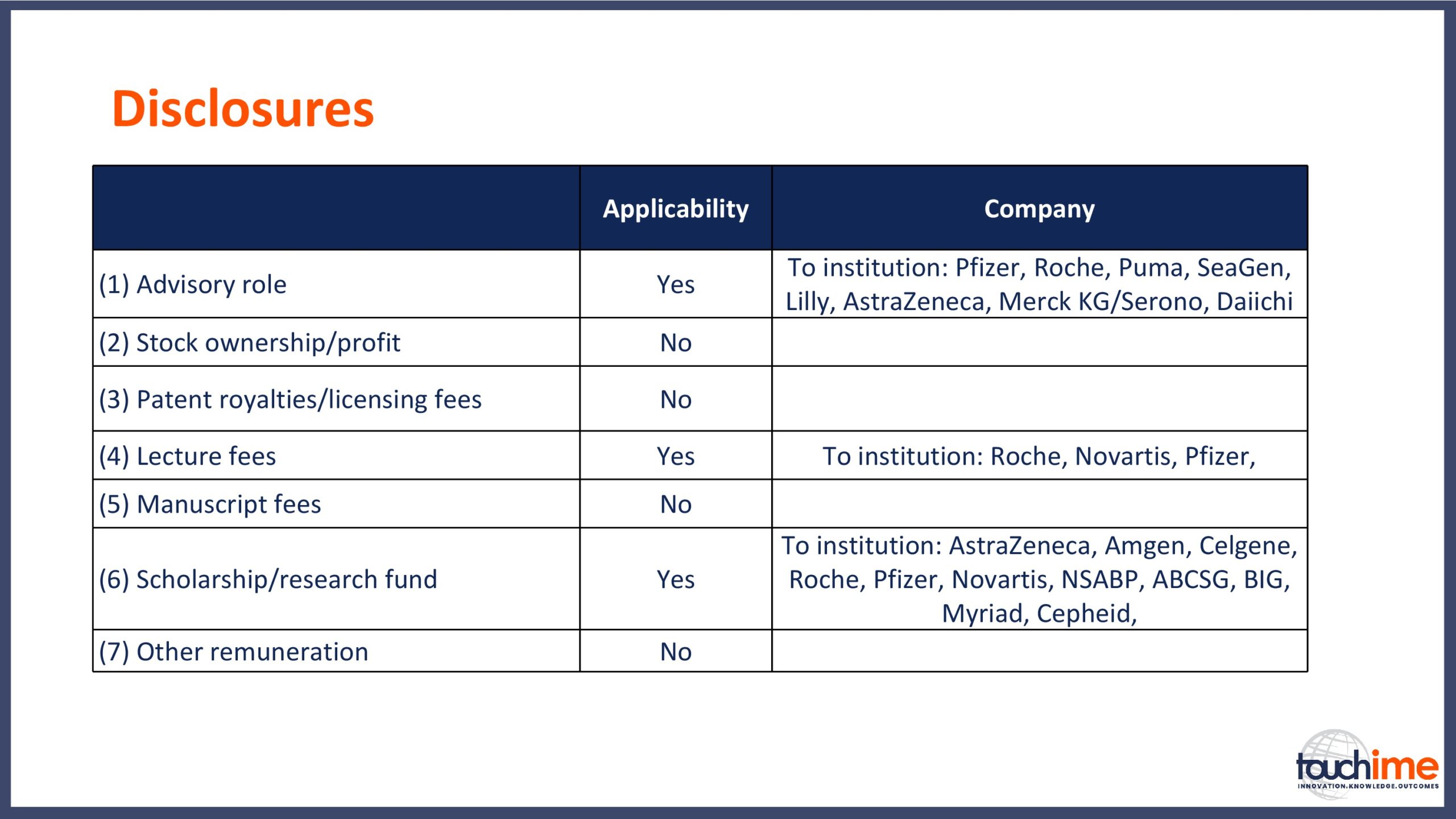
Prof. Sibylle Loibl discloses: Honoraria received by my institution from AbbVie, Amgen, AstraZeneca, Celgene,Daiichi, Novartis, Pfizer, PRIME, Roche, Seattle Genetics, Teva and Vifor. Research grants received by my institution from ABGSG, AbbVie, Amgen, AstraZeneca, BIG, Celgene, Cepheid, Myriad, NSABP, Novartis, Pfizer, Roche, Teva and Vifor.
Prof. Gabriel Hortobagyi, Professor in the Department of Breast Medical Oncology at The University of Texas M.D. Anderson Cancer Center, provides his expert insight into key data presented at the ESMO Breast Cancer Congress 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Joan Albanell, Head of Medical Oncology at Hospital del Mar, Barcelona, Spain, provides his expert insight into key data presented at the ESMO Breast Cancer Congress 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Sibylle Loibl, Associate Professor at the University of Frankfurt and Chief Executive Officer and Chair of the German Breast Group, provides her expert insight into key data presented at the ESMO Breast Cancer Congress 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Mario Campone, CEO of the Institut de Cancérologie de l’Ouest, France provides his expert insight into key data presented at the ESMO Breast Cancer Congress 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Guiseppe Curigliano, Associate Professor of Medical Oncology at the University of Milan and the Head of the Division of Early Drug Development at European Institute of Oncology, Italy, provides his expert insight into key data presented at the ESMO Breast Cancer Congress 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Prof. Stephen Johnston, Professor of Breast Cancer Medicine and Consultant Medical Oncologist at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London, provides his expert insight into key data presented at the ESMO Breast Cancer Congress 2019 and discusses what the latest developments in CDK4/6 inhibitors mean for optimizing clinical outcomes for patients with advanced breast cancer.
Please Select A Video:
Overview & Learning Objectives
Overview
Watch a series of internationally renowned clinical specialists from Europe and the United States discuss key clinical data of interest from the European Society for Medical Oncology (ESMO) Breast Cancer Congress, Berlin, Germany, 2–4 May 2019.
The potential implications of these data for optimizing clinical outcomes for patients with advanced breast cancer are explored from both global and regional perspectives.
The information in this activity is intended for oncologists, nurses, and other healthcare professionals involved in the treatment of patients with breast cancer.
Learning Objectives
After watching this touchCONGRESS, you should be able to:
- Describe the role of CDK4/6 inhibitors in the context of the current and evolving treatment landscape for patients with HR+/HER2- advanced breast cancer
- Evaluate the importance of selecting the optimal treatment based on the individual patient, and the challenges around subsequent sequencing of therapy
- Summarize the importance of managing the safety profiles of CDK4/6 inhibitor therapy, and recognise the significance of the multidisciplinary team in optimizing patient outcomes and maintaining on-treatment benefits

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights