touchCONGRESS HR+/HER2- Advanced breast cancer: what are the latest developments in CDK4/6 inhibition from the SABCS 2019?
Hear expert review and interpretation of the latest findings for treating HR+/HER2- advanced breast cancer with CDK4/6 inhibitors in this webinar from the San Antonio Breast Cancer Symposium (SABCS), Texas, US, 10–14 December 2019.
Part 1: Watch internationally renowned expert Dr Fátima Cardoso review key data from SABCS 2019
Part 2: Choose from leading experts who discuss what the data finding mean for global and regonal practice
Introduction and summary of the key trials for CDK4/6 inhibitors
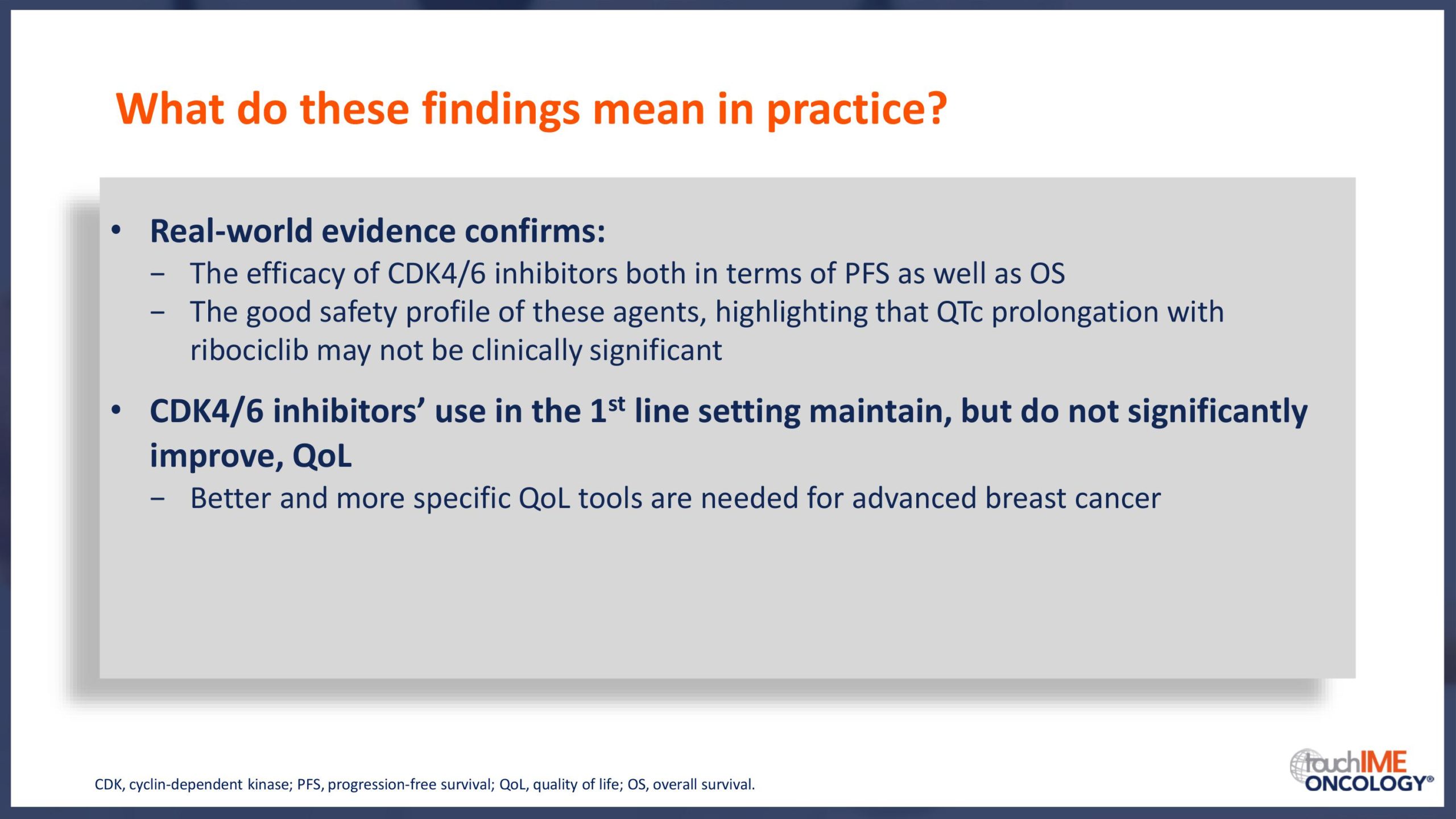
SABCS 2019 – Efficacy and safety – Extended and subgroup analyses
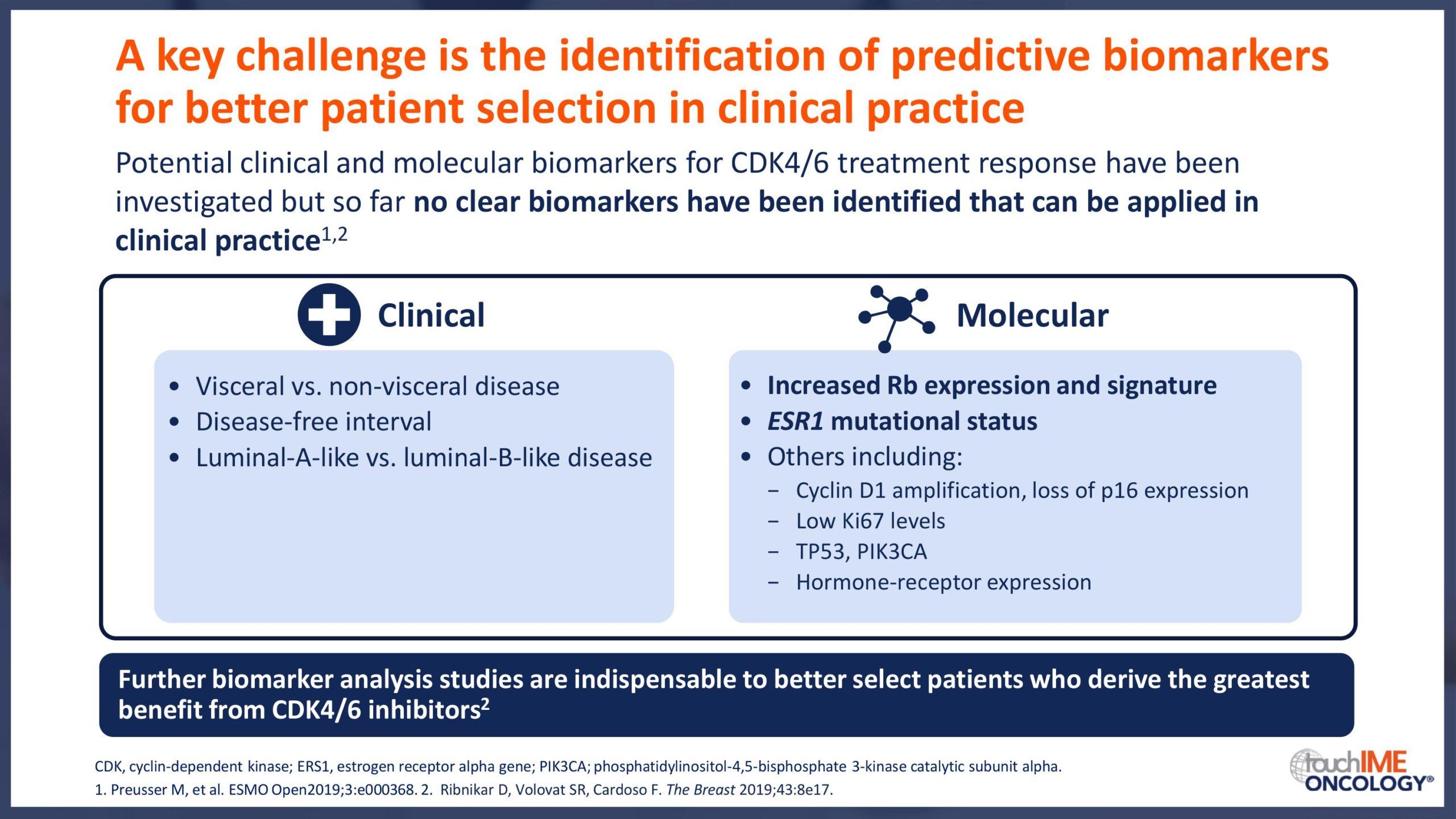
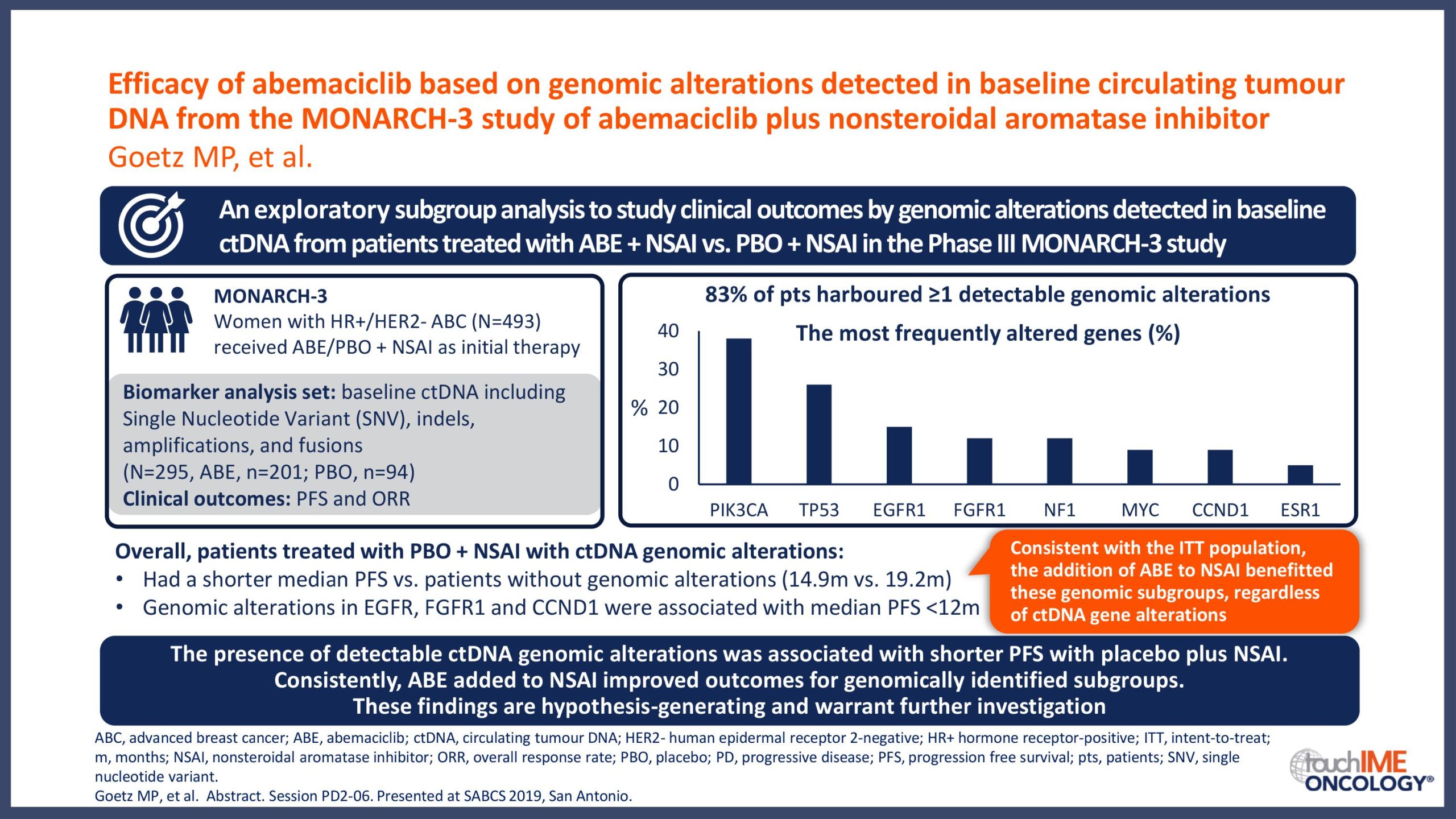
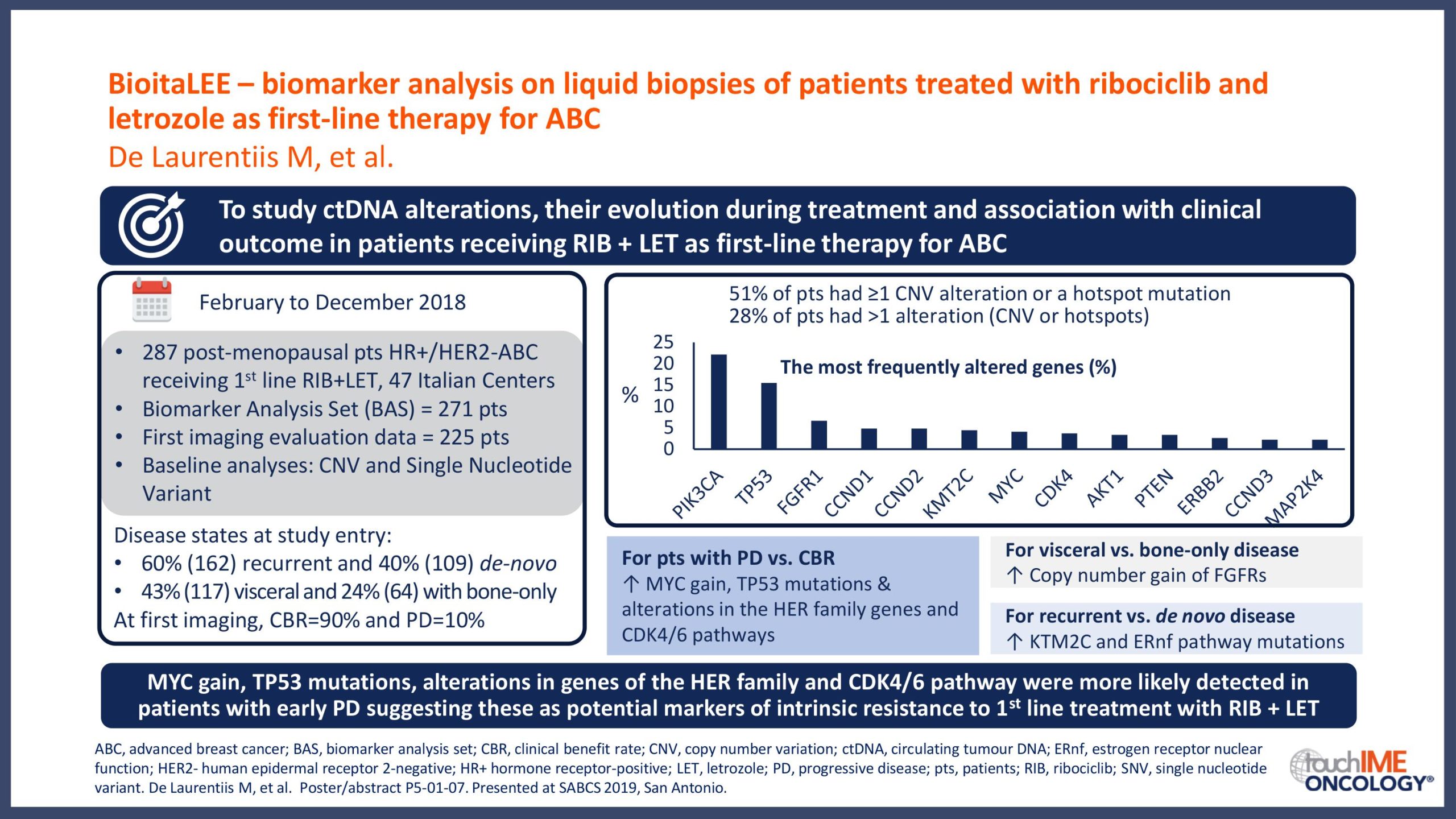
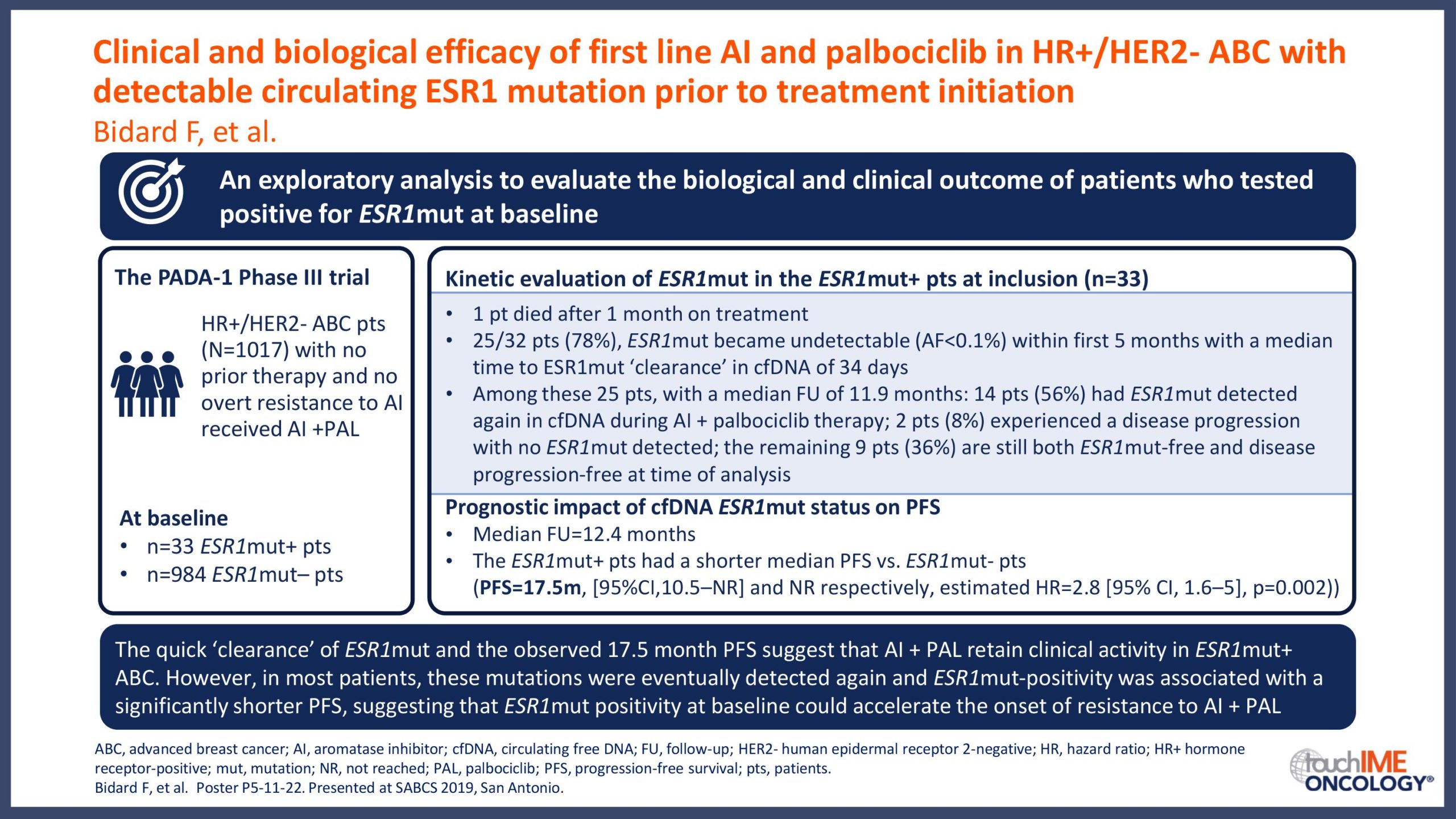
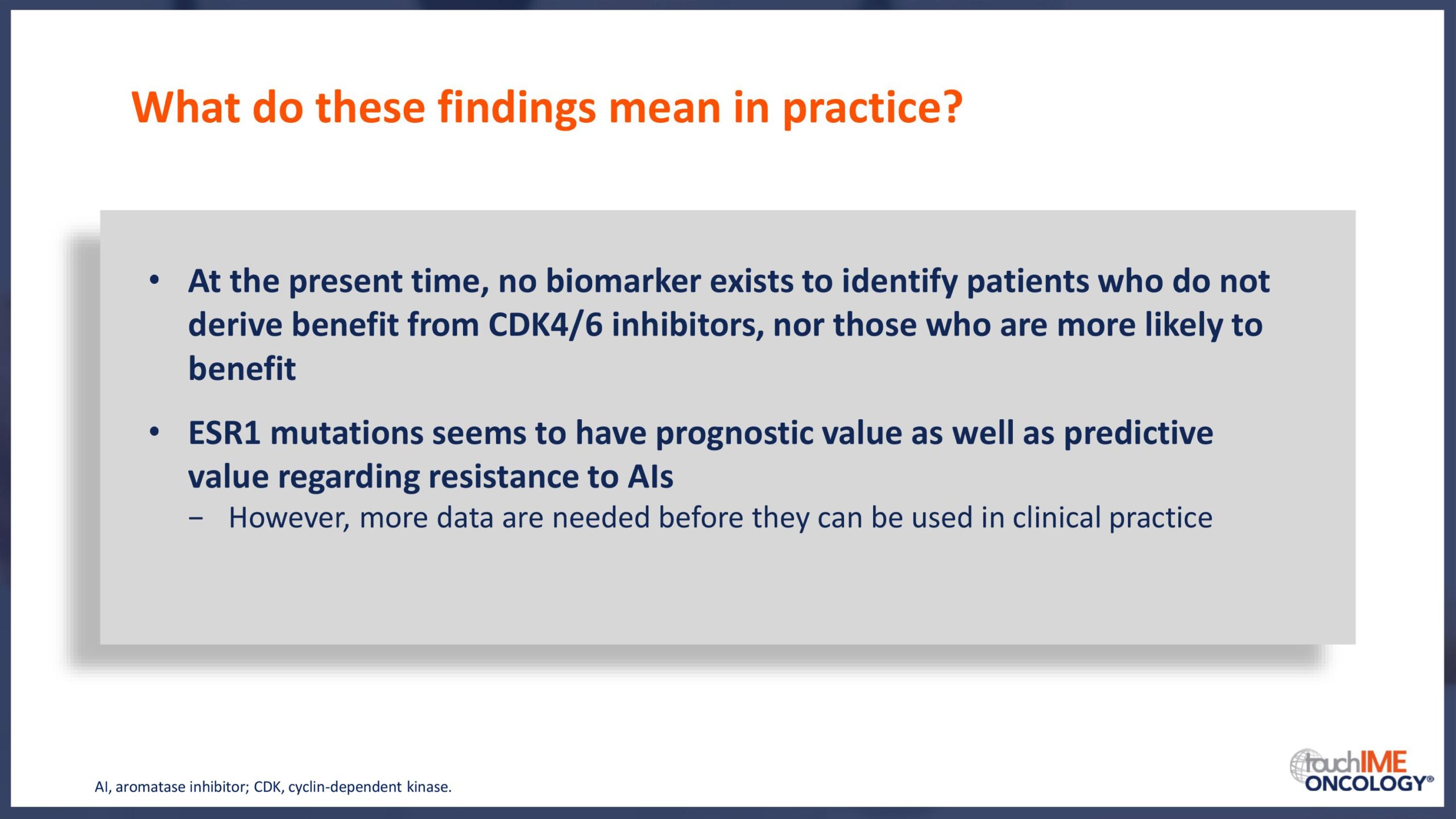
SABCS 2019 – Biomarkers and resistance – What does the research show?
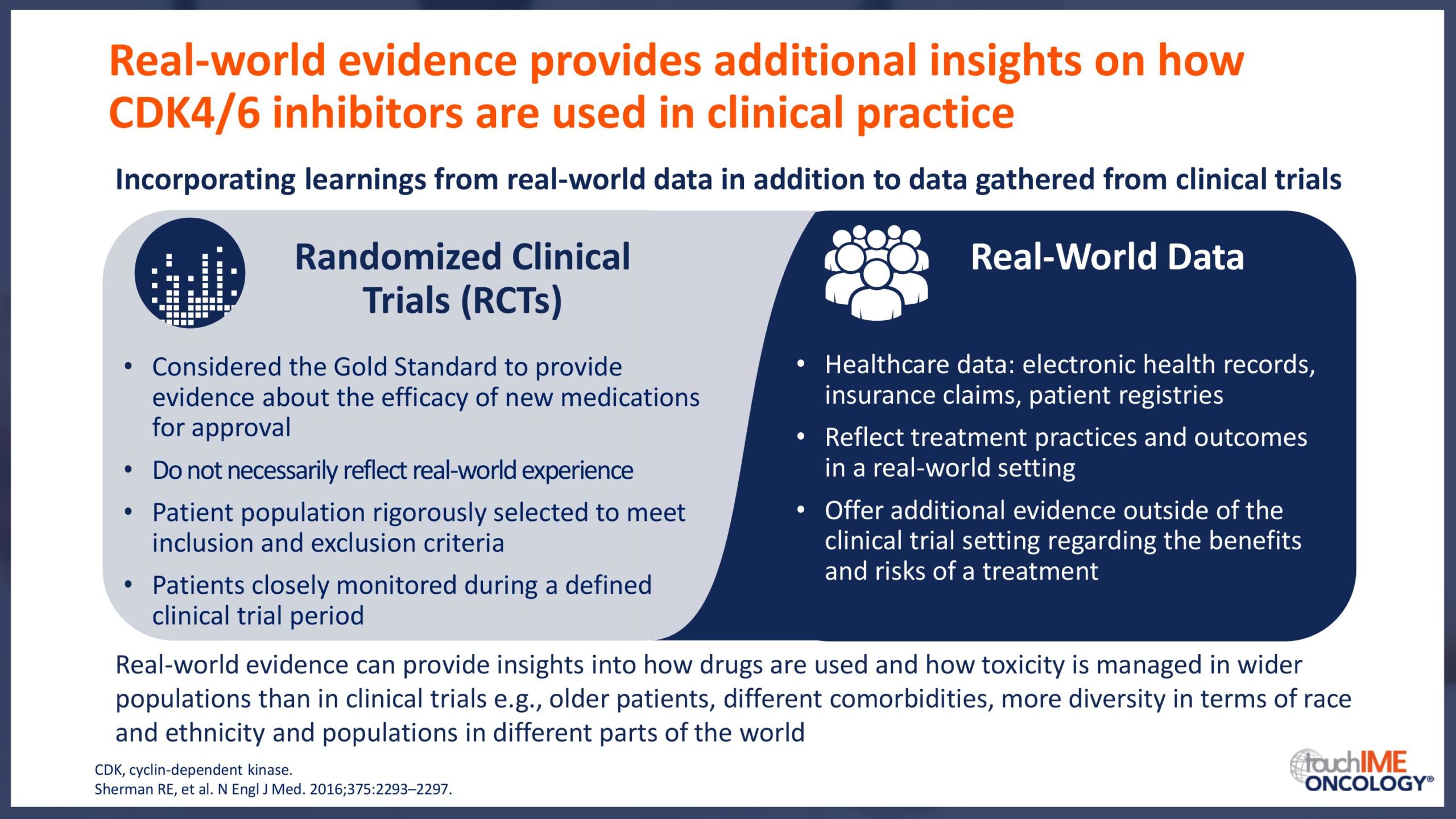
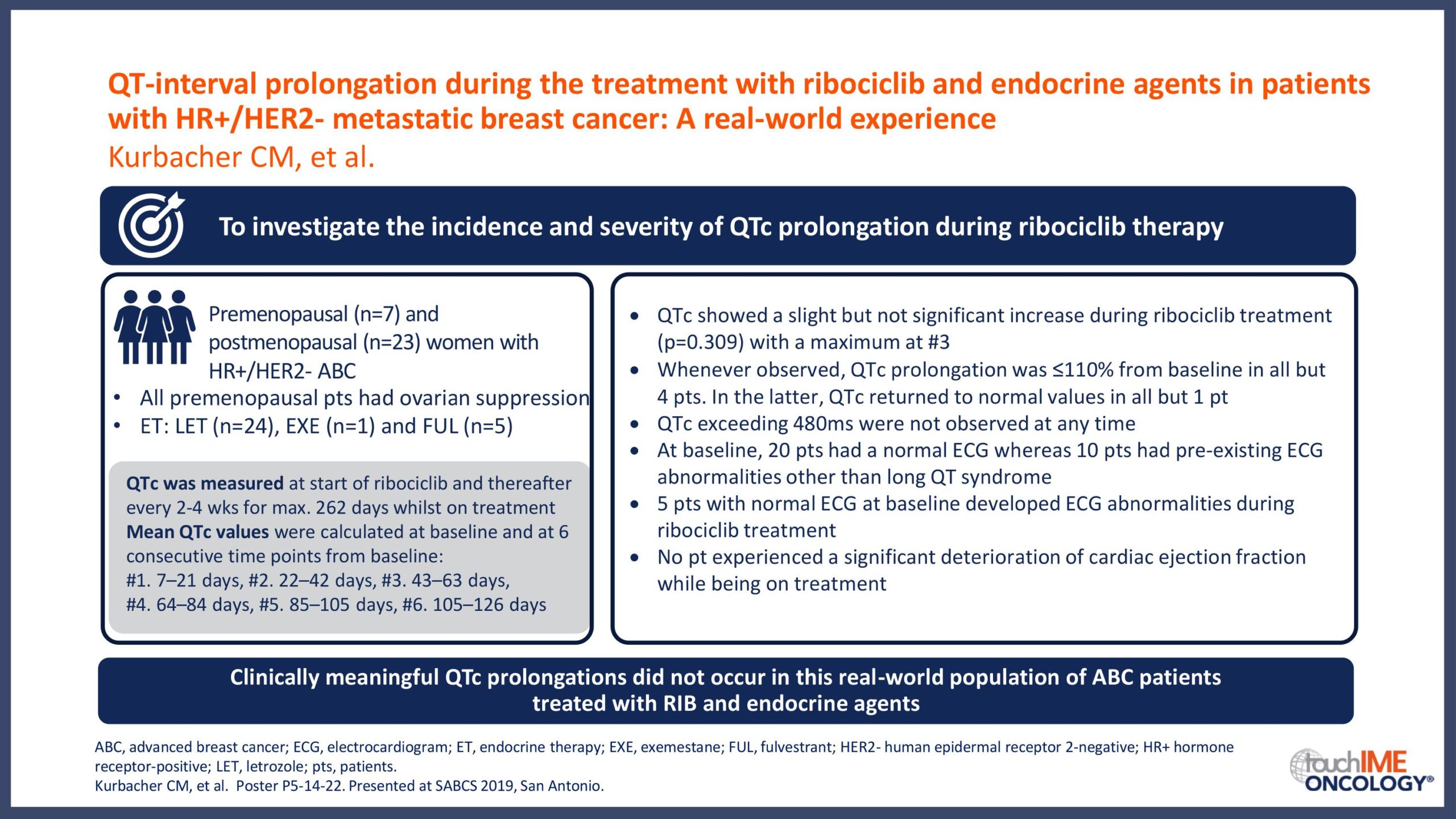
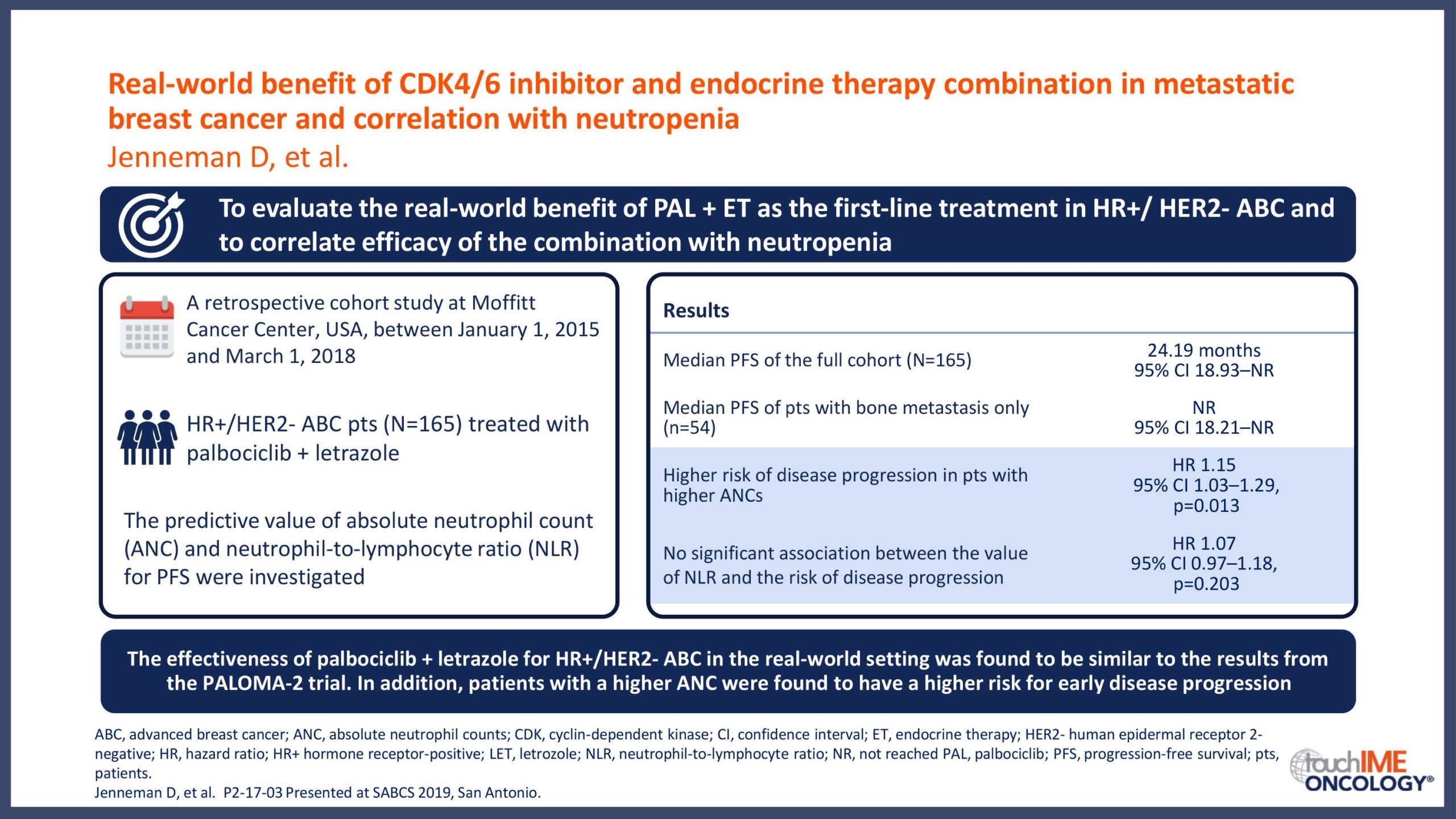
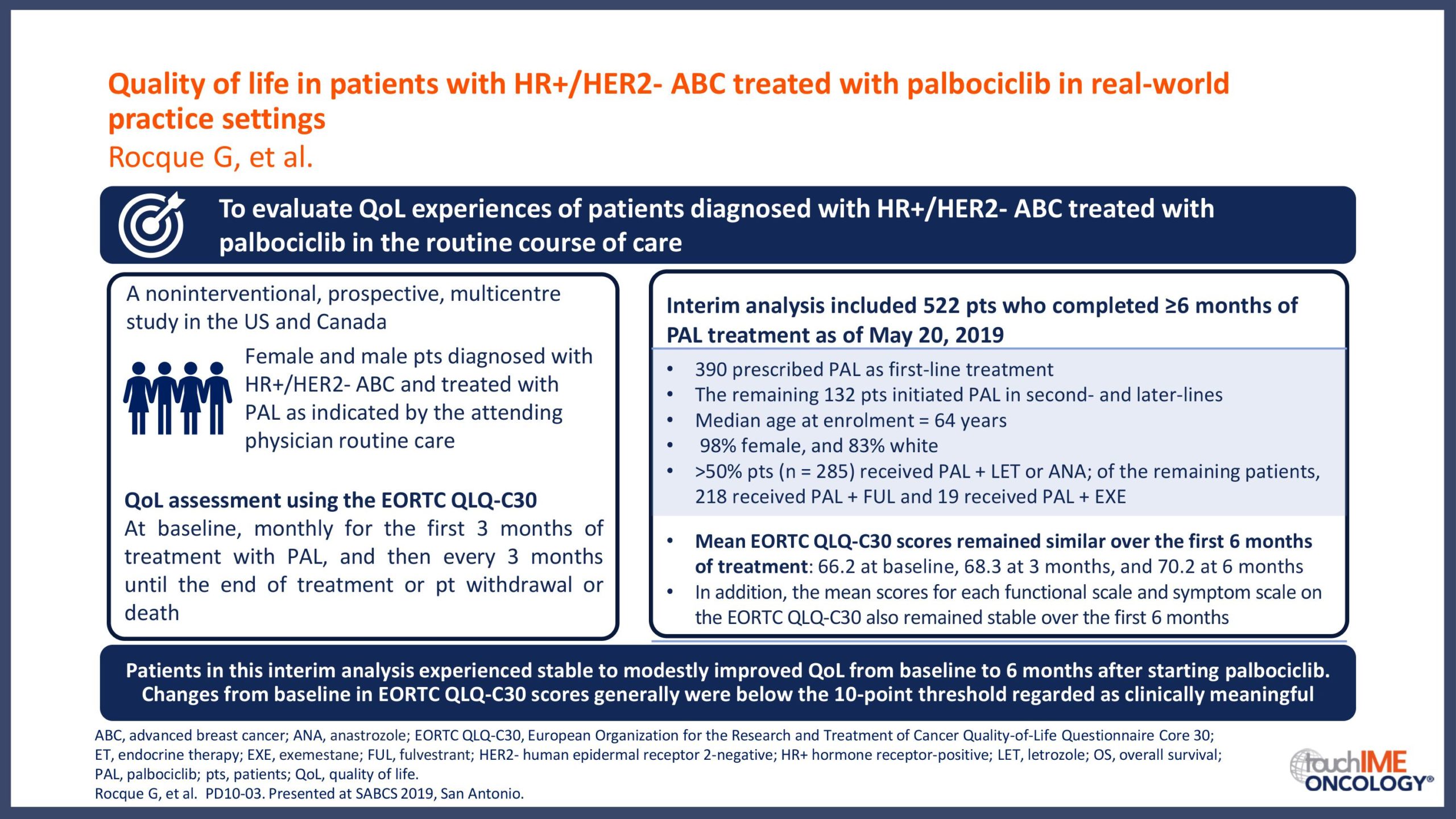
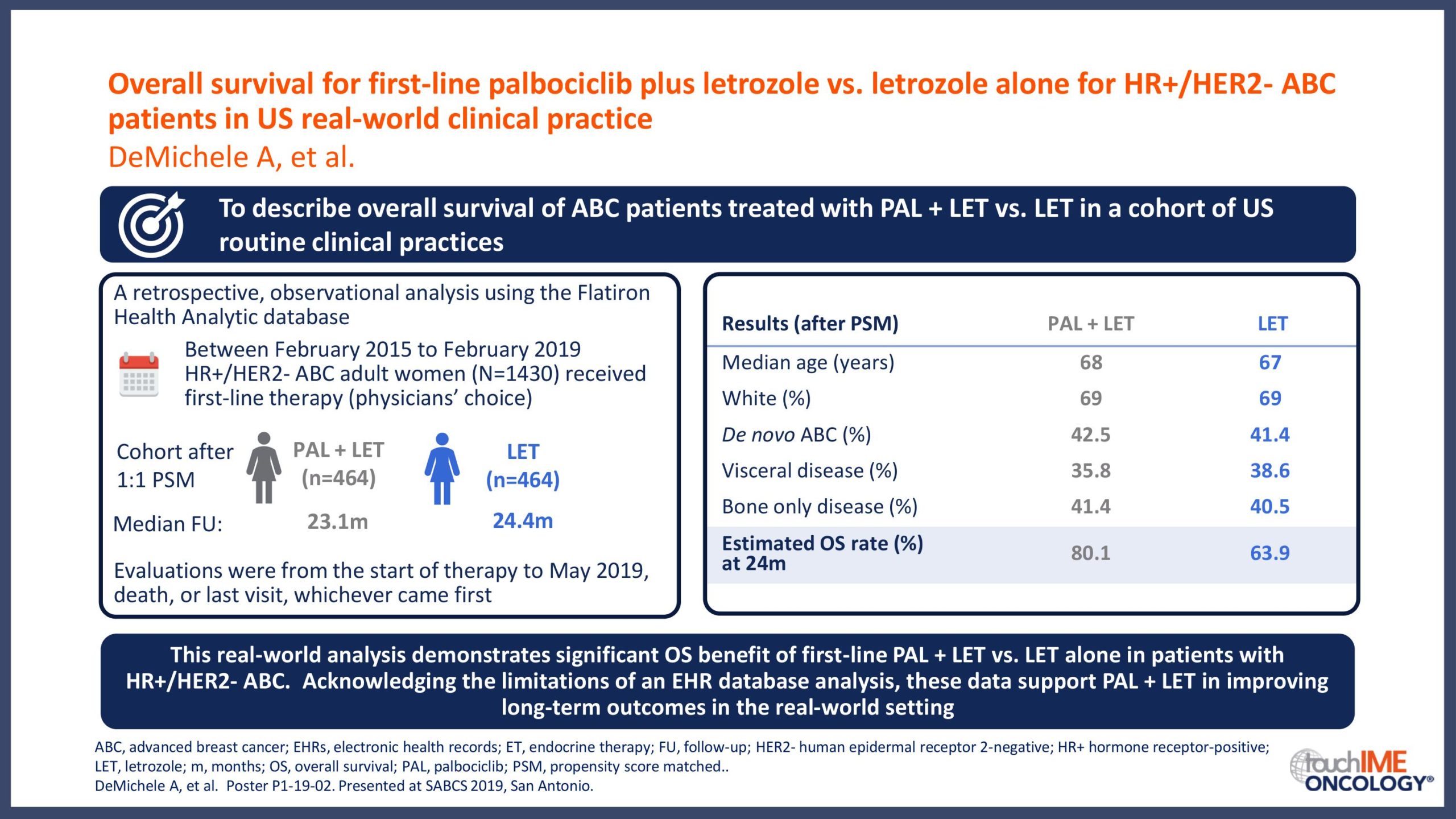
SABCS 2019 – Real-world and practical insights for CDK4/6 inhibitors
Overview
Watch Fátima Cardoso consider the key research findings from SABCS 2019 and discusses the optimal use of CDK4/6 inhibitors for patients with HR+/HER2- advanced breast cancer, including:
- What are the latest data for CDK4/6 inhibitors in patients with HR+/HER2- advanced breast cancer? Exploring extended and sub group analyses for further insights
- Research for biomarkers for CDK4/6 treatment response that can be applied in clinical practice continues. What do the latest results show?
- How do the benefits of CDK4/6 inhibitors translate to the daily clinic? Focus on evidence from real-world studies
Dr Fátima Cardoso is the Director of the Breast Unit of the Champalimaud Clinical Center in Lisbon, Portugal. Dr Cardoso’s research interests include biology of breast cancer, prognostic and predictive markers of response to systemic therapy and new anticancer agents. She is actively involved in a number of phase I-III breast cancer clinical trials for which she has received grants from a variety of foundations and societies, as well as serving as the scientific director of the international research network TRANSBIG. Dr Cardoso is actively involved in numerous professional organizations such as ESMO, EORTC, ASCO, and AACR where she serves on several committees. She is also the Breast Cancer Program Coordinator of the European School of Oncology and chair of the Advanced Breast Cancer International Consensus Guidelines Conference. Dr Cardoso is editor-in-chief of The Breast Journal, associate editor of the European Journal of Cancer, and an editorial board member of several other journals. She was awarded the prestigious Order of Santiago da Espada for Scientific Merit, from the President of Portugal in 2015. Dr Cardoso has authored about 300 publications and has presented her work nationally and internationally.
Dr Fátima Cardoso discloses: Consultancy role for Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Medscape, Merck Sharp & Dohme, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Samsung Bioepis, Seattle Genetics and Teva. Clinical trial funding (to the institute) from Amgen, Astra-Zeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Eisai, Fresenius GmbH, Genentech, GlaxoSmithKline, Ipsen, Incyte, Nektar Therapeutics, Nerviano, Novartis, Macrogenics, Medigene, MedImmune, Merck, Millenium, Pfizer, Pierre-Fabre, Roche, Sanofi-Aventis, Sonus, Tesaro, Tigris, Wilex and Wyeth.
Dr Eva M. Ciruelos, Medical Oncologist and Coordinator of the Multidisciplinary Breast Cancer Unit at the University Hospital 12 de Octubre in Madrid, Spain, considers the impact of emerging data presented at the SABCS 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Prof. George Sledge from the Department of Medicine, Stanford University School of Medicine, Stanford, California, US, considers the impact of emerging data presented at the SABCS 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Dr Norikazu Masuda from the National Hospital Organization Osaka National Hospital, Osaka, Japan considers the impact of emerging data presented at the SABCS 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Dr Fátima Cardoso, Director of the Breast Unit of the Champalimaud Clinical Center in Lisbon, Portugal, considers the impact of emerging data presented at the SABCS 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Prof. Frédérique Penault-Llorca, Chief Executive Officer of the Jean-Perrin Comprehensive Cancer Center, Clermont-Ferrand, France, considers the impact of emerging data presented at the SABCS 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer
Prof. Dr Jens Huober, Director of the Department of Obstetrics and Gynaecology of the University of Ulm, Germany, considers the impact of emerging data presented at the SABCS 2019 and discusses how they may influence treatment decisions for patients with HR+/HER2- advanced breast cancer.
Please Select A Video:
Overview & Learning Objectives
Overview
Hear expert review and interpretation of the latest findings for treating HR+/HER2- advanced breast cancer with CDK4/6 inhibitors in this webinar from the San Antonio Breast Cancer Symposium (SABCS), Texas, US, 10–14 December 2019.
The information in this activity is intended for oncologists, nurses, and other healthcare professionals involved in the treatment of patients with breast cancer.
Learning Objectives
After watching this touchCONGRESS, you should be able to:
- Describe the role of CDK4/6 inhibitors in the context of the current and evolving treatment landscape for patients with HR+/HER2- advanced breast cancer
- Evaluate the importance of selecting the optimal treatment based on the individual patient, and the challenges around subsequent sequencing of therapy
- Summarize the importance of managing the safety profiles of CDK4/6 inhibitor therapy, and recognize the significance of the multidisciplinary team in optimizing patient outcomes and maintaining on-treatment benefits

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights