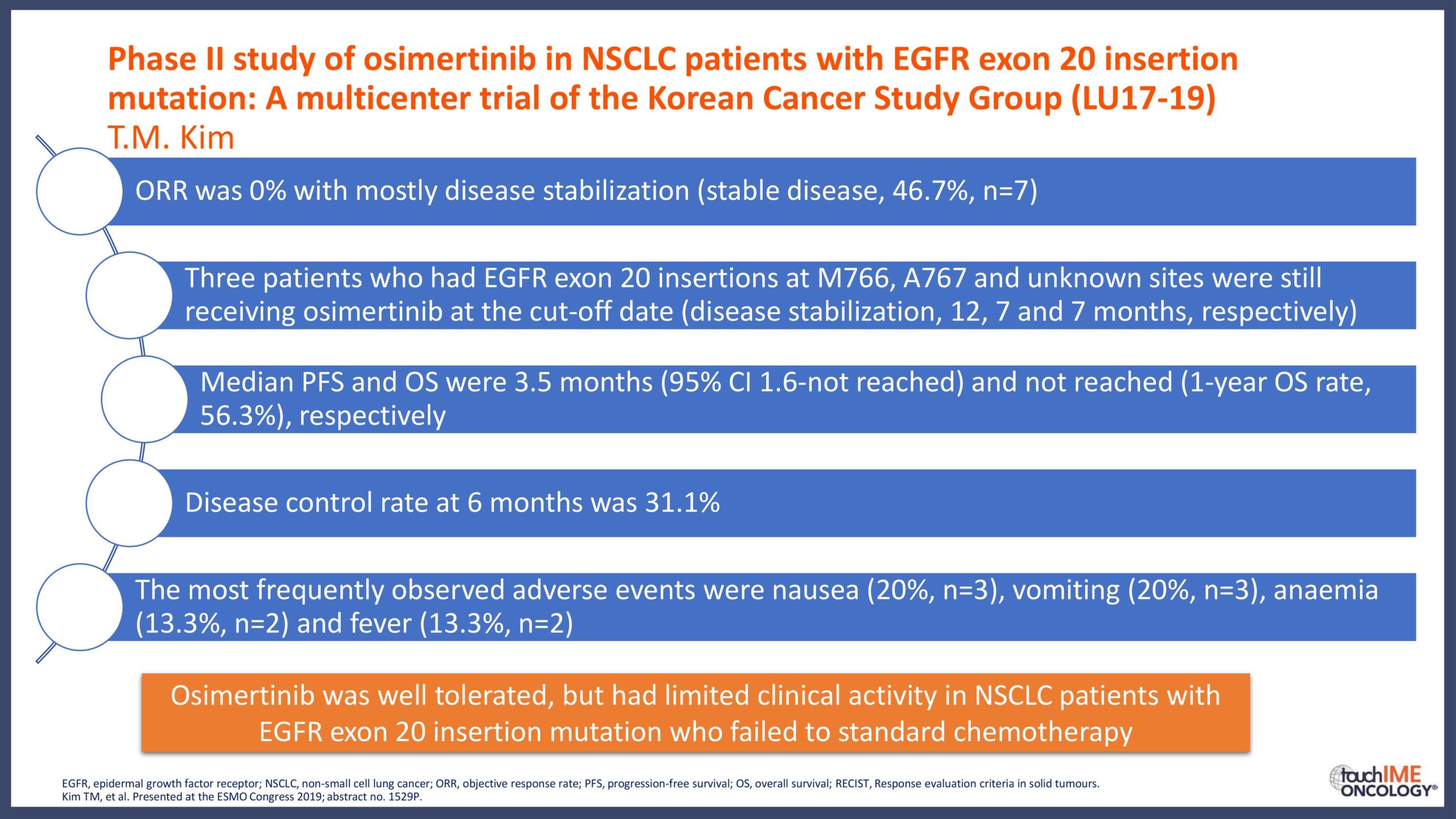
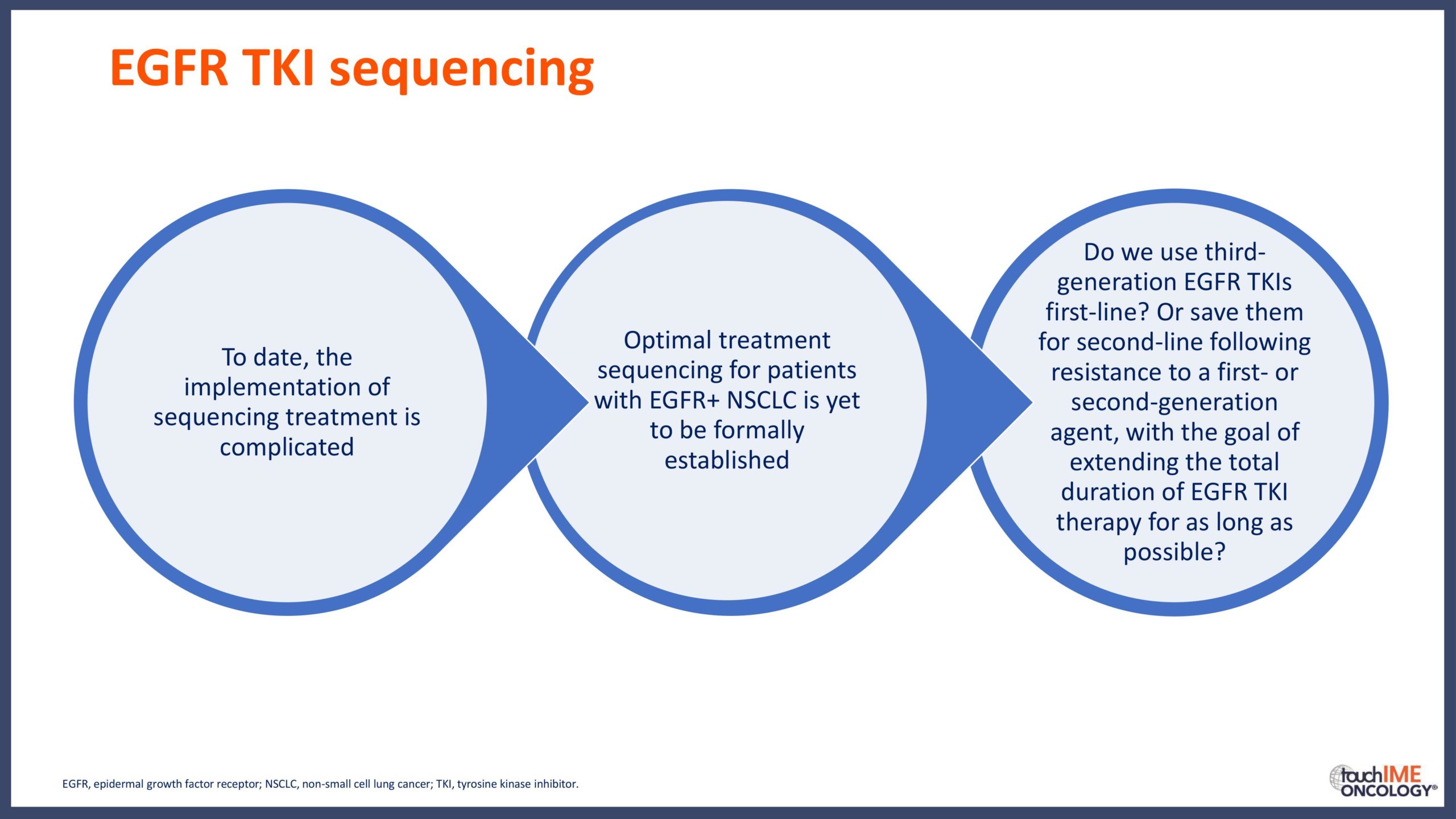
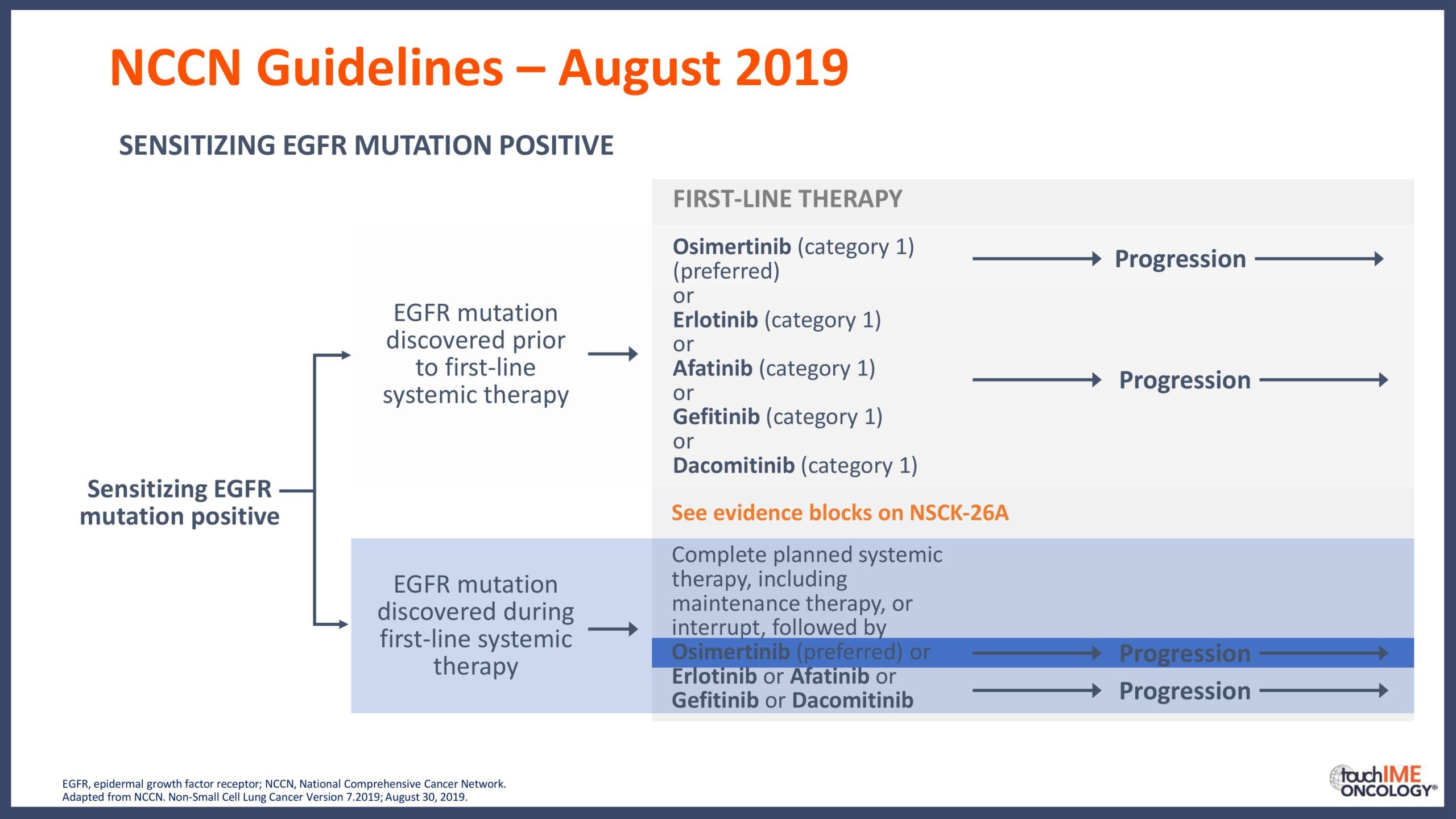
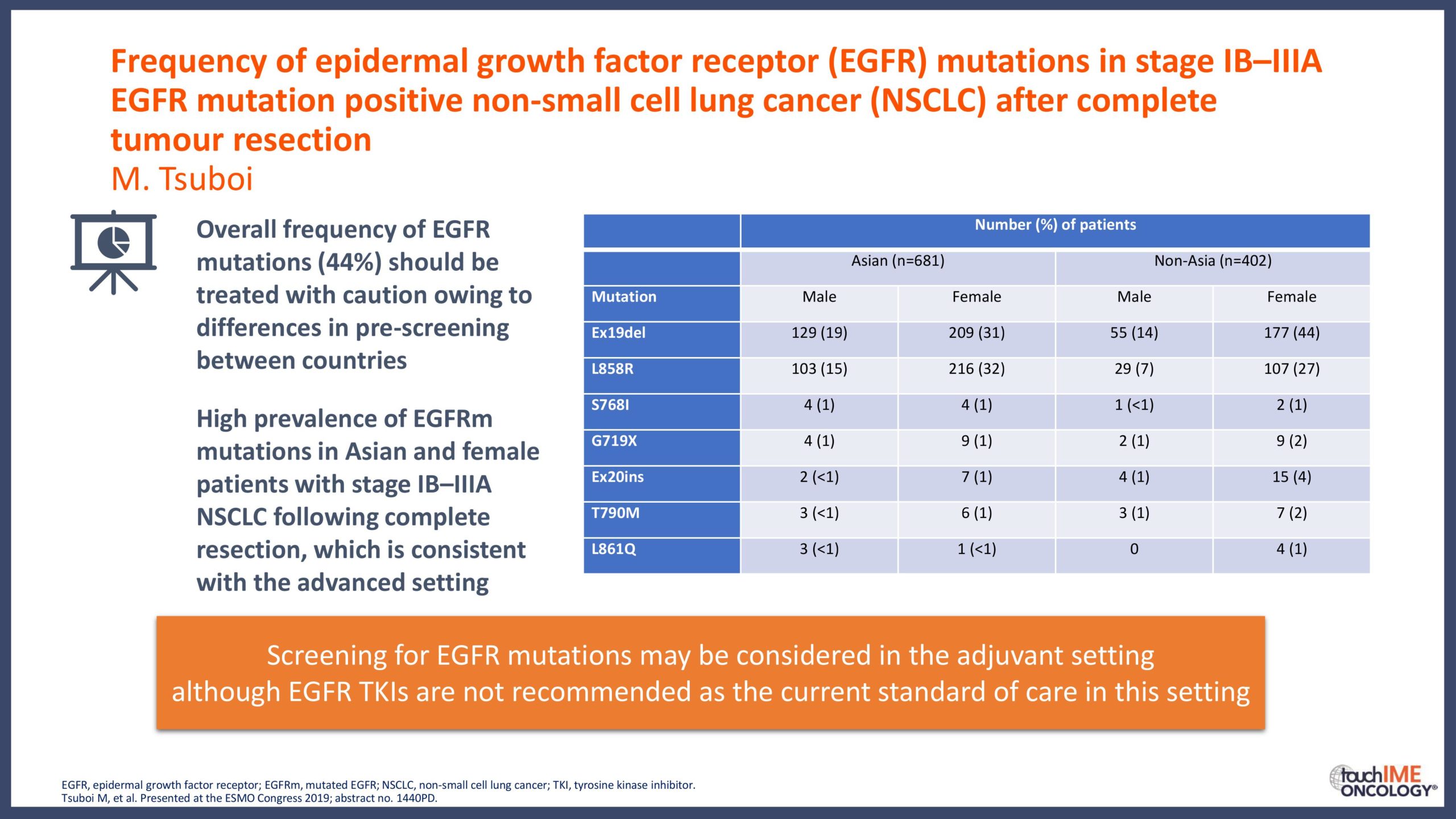
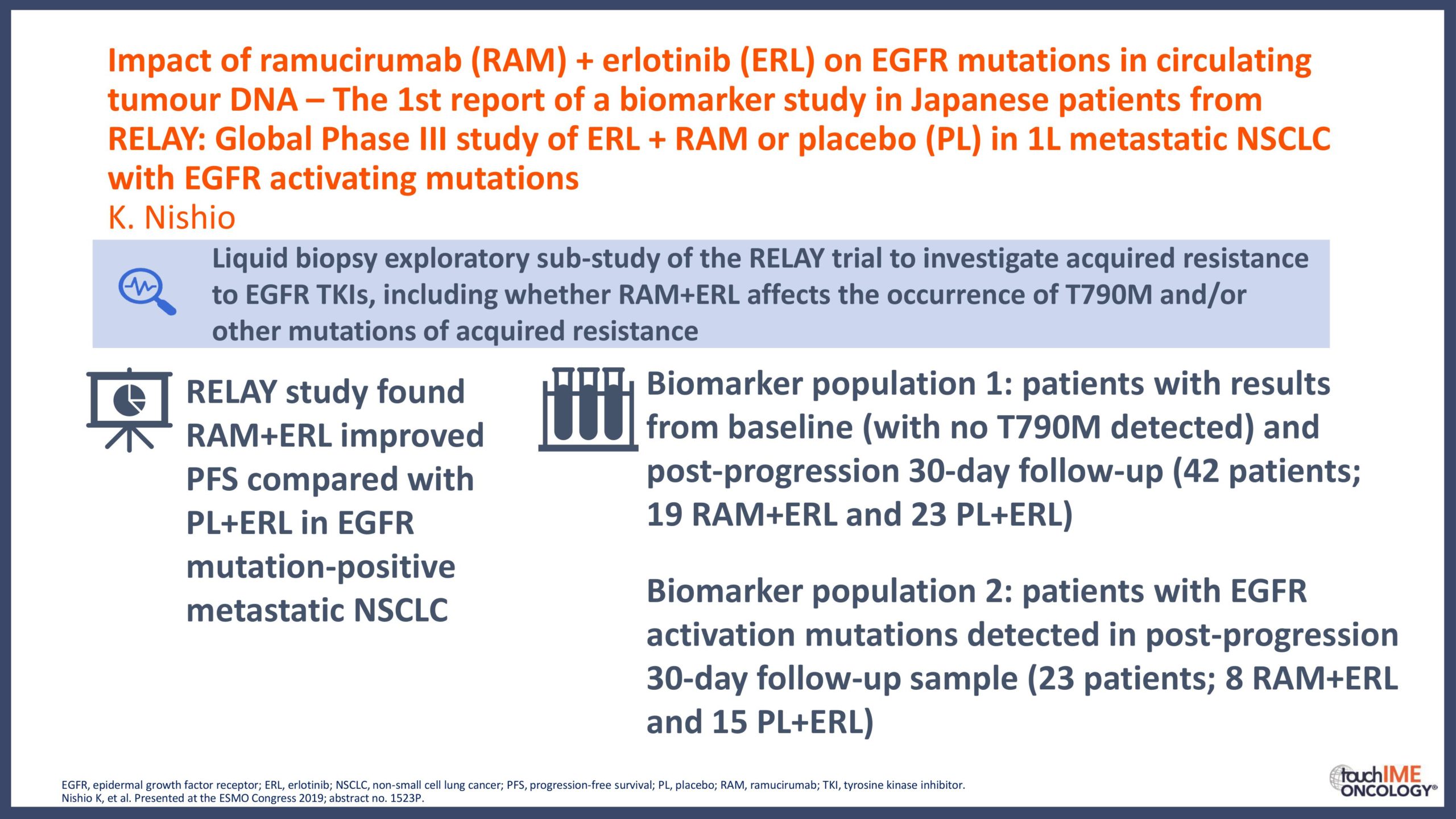
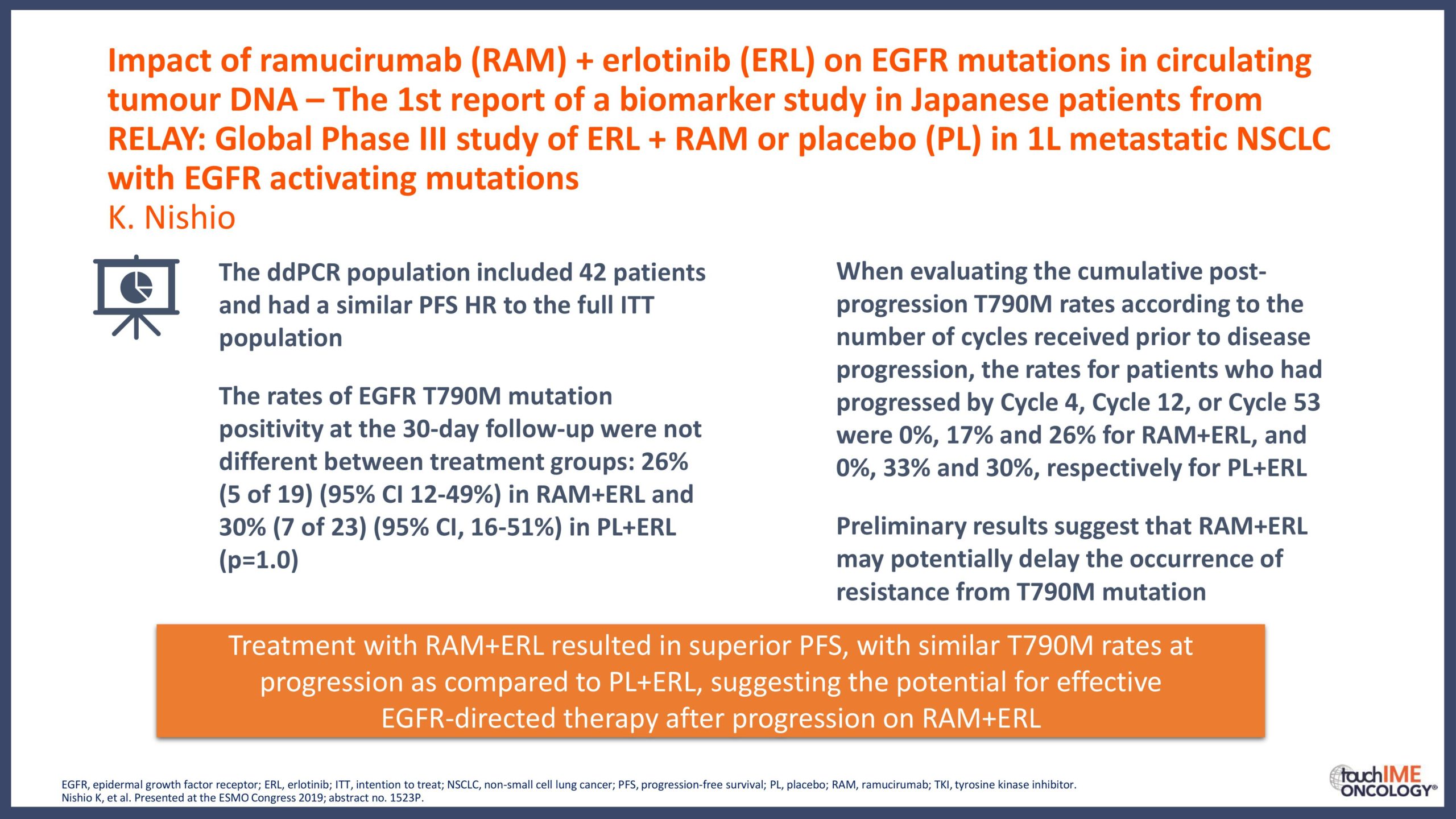
touchCONGRESS Should we use third-generation TKIs up front in EGFR+ NSCLC?
Stay up to date with the latest developments in the management and treatment of patients with EGFR+ NSCLC with our expert summary from the ESMO Congress 2019 in Barcelona, Spain, 27 September to 1 October 2019.
Part 1: Watch internationally renowned expert Prof. Suresh Ramalingam review key data from the ESMO Congress
Part 2: Choose from leading experts who discuss what the data findings mean for global and regional practice
Introduction
What are the key clinical data for EGFR TKIs that will inform daily practice?
Can we determine an optimal sequencing of treatment for patients with EGFR+ NSCLC?
How can we tailor care to the individual
Watch Suresh Ramalingam reviewing the most important emerging data presented at the ESMO Congress 2019 and discussing their potential impact for addressing real-life clinical unmet needs in patients with EGFR+ NSCLC, including:
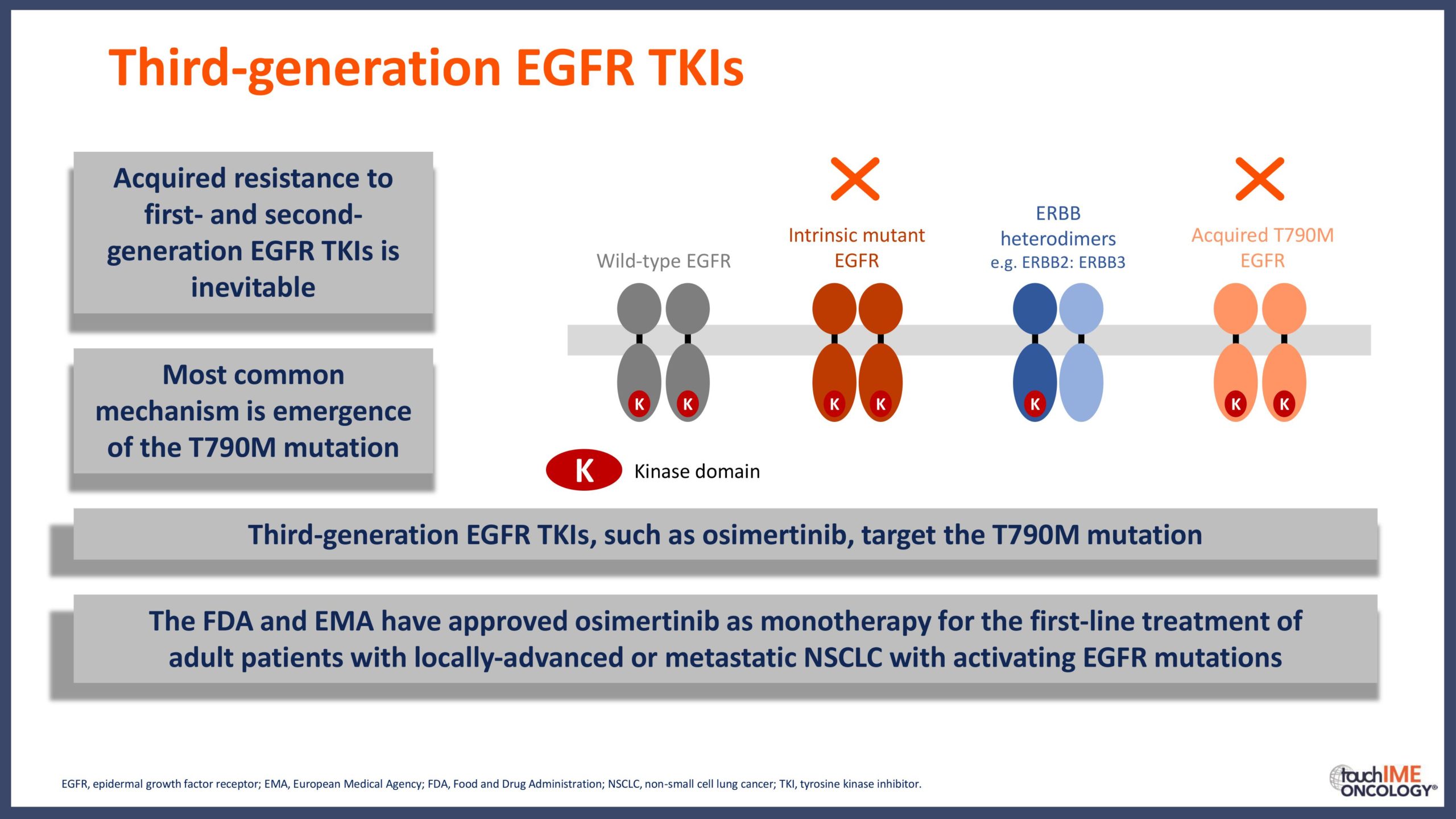
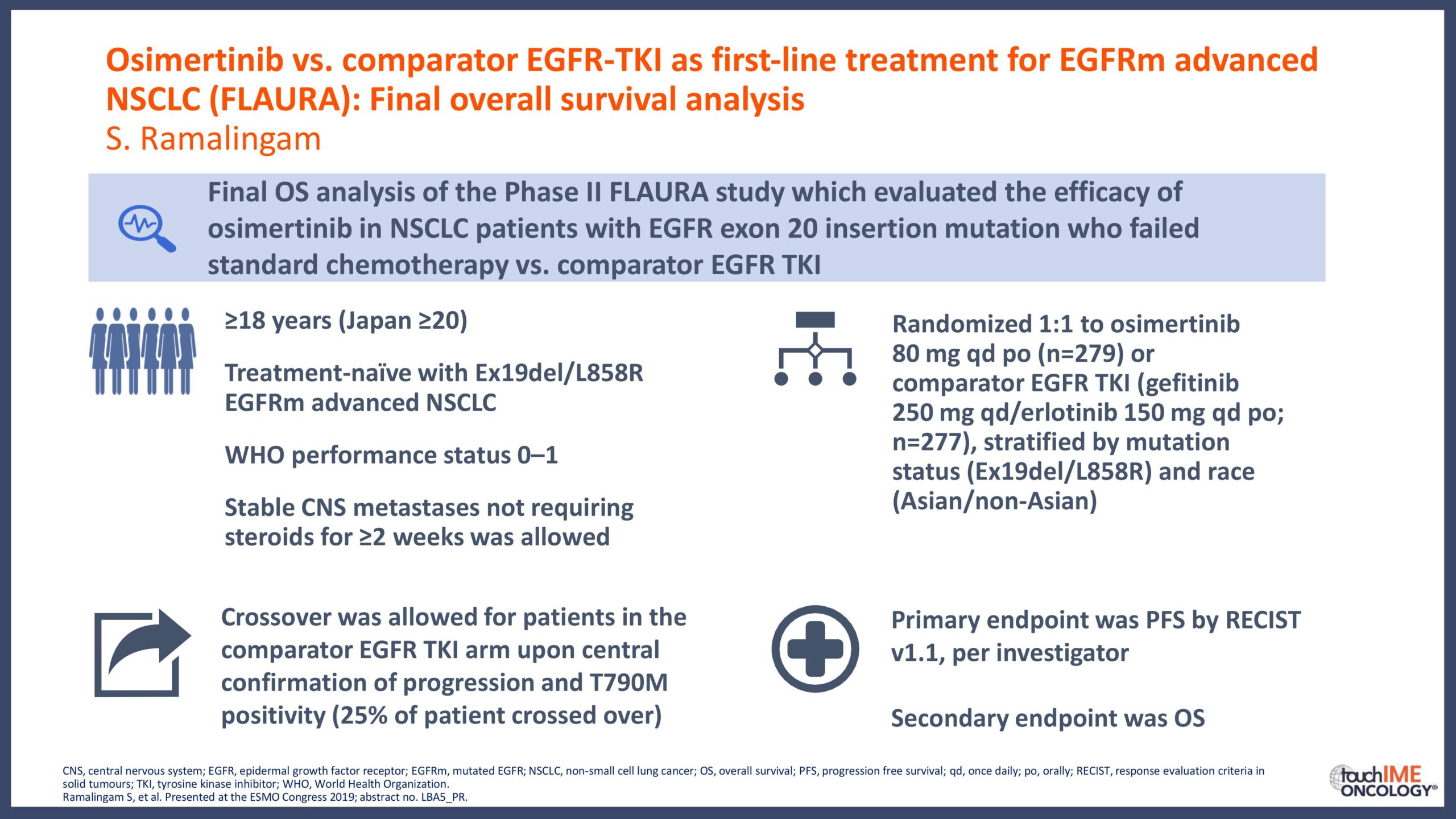
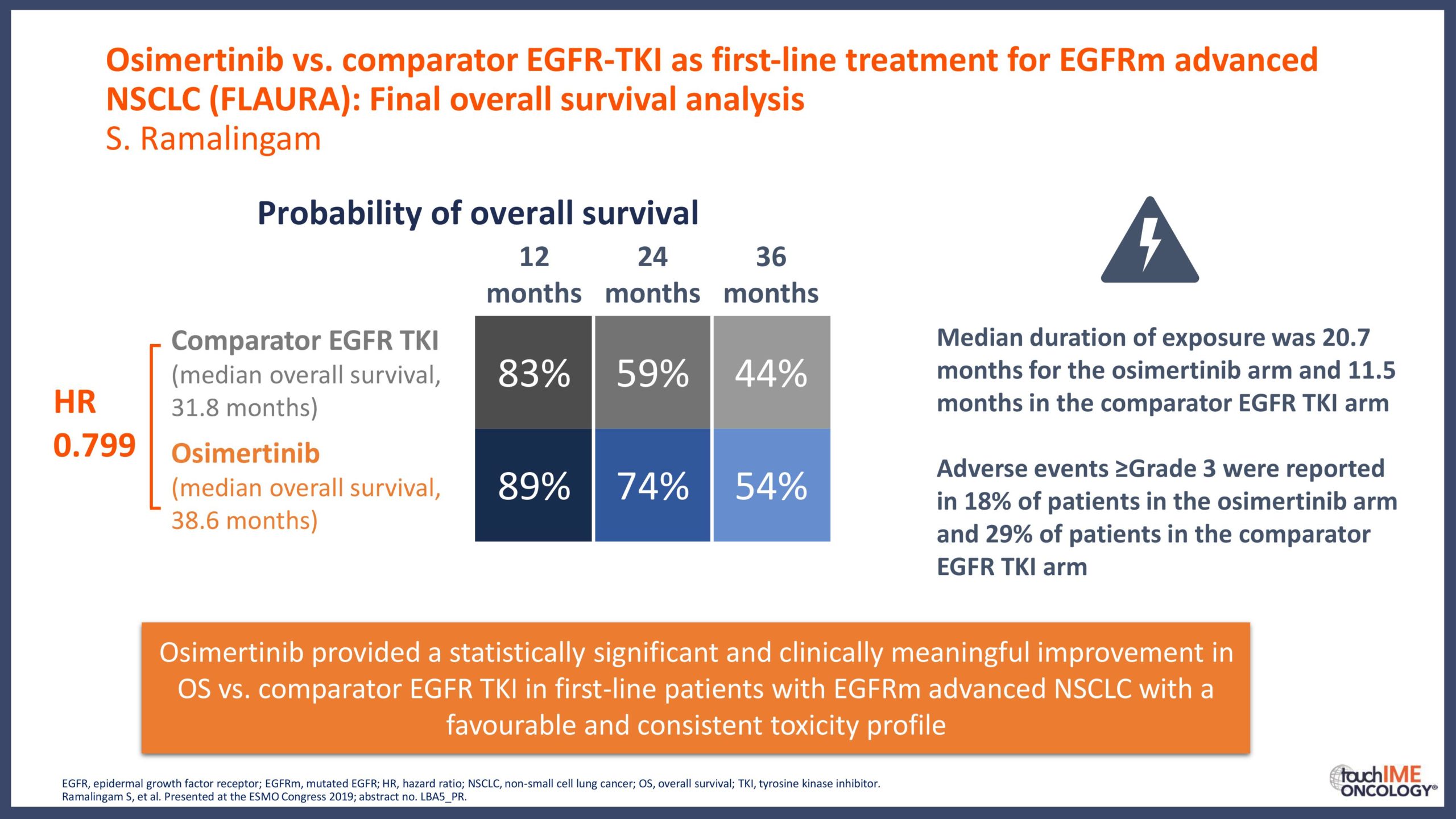
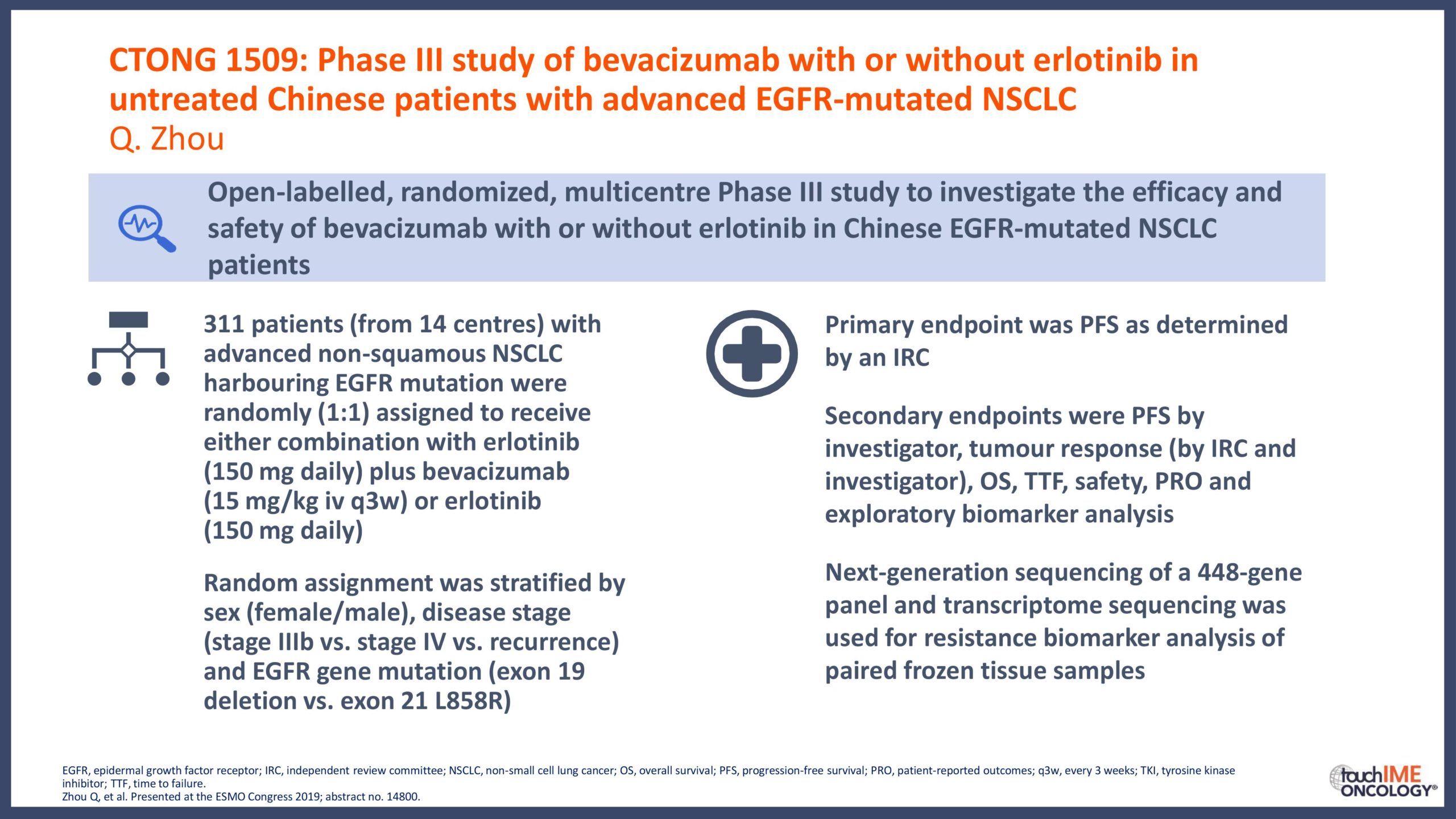
- What are the key clinical data for EGFR TKIs that will inform daily practice? Focus on the different efficacy and safety profiles of third-generation agents, compared with first- and second-generation TKIs
- Can we determine an optimal sequencing of treatment for patients with EGFR+ NSCLC? Focus on third-generation EGFR TKIs with earlier-generation agents and treatment options following resistance to third-generation inhibitors
- How does real-world evidence inform clinical practice? Focus on how patients are treated currently and the barriers to maximizing patient outcomes
Suresh Ramalingam is Professor of Hematology and Medical Oncology and Director of the Division of Medical Oncology at the Winship Cancer Institute, Emory University School of Medicine, Atlanta, GA, USA.
Prof. Ramalingam serves as the Chair of the ECOG-ACRIN Thoracic Malignancies Committee. He also serves on the Editorial Board of leading cancer journals such as the Journal of Clinical Oncology, Cancer, Annals of Oncology and Clinical Lung Cancer.
Prof. Ramalingam’s research interests include the development of novel anti-cancer agents and evaluation of methods to individualize therapies for patients. He has conducted several clinical trials with molecularly targeted agents in the treatment of small cell and non-small cell lung cancer.
Prof. Ramalingam discloses: Consultancy role with AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Genentech, Merck and Takeda. Research funding from Advaxis, Amgen, AstraZeneca, Bristol Myers Squibb, Merck and Tesaro.
Prof. James Yang, Director of the Department of Oncology at the National Taiwan University, Taiwan, provides his expert insight into key data presented at the ESMO 2019 Asia Congress and discusses the latest developments in EGFR TKIs for optimizing outcomes for patients with NSCLC.
Prof. Keunchil Park, Professor, Division of Hematology-Oncology at the Samsung Medical Center, Seoul, Korea, provides his expert insight into key data presented at the ESMO Asia 2019 Congress and discusses the latest developments in EGFR TKIs for optimizing outcomes for patients with NSCLC
Prof. Suresh Ramalingam, Professor of Hematology and Medical Oncology at the Emory University School of Medicine, Atlanta, USA, provides his expert insight into key data presented at the ESMO 2019 Congress and discusses the latest developments in EGFR TKIs for optimizing outcomes for patients with NSCLC.
Prof. Frank Griesinger, Director of the Department of Haematology and Oncology at the Pius-Hospital, Oldenburg, lecturer at the University of Göttingen and Professor of Oncology at the University of Oldenburg, Germany, provides his expert insight into key data presented at the ESMO 2019 Congress and discusses the latest developments in EGFR TKIs for optimizing outcomes for patients with NSCLC.
Prof. Liyan Jiang, Director of the Office for National Agency for Clinical Trials of Drugs at the Shanghai Chest Hospital, Deputy Director of the Clinical Research Office of Lung Cancer at the Shanghai Chest Tumour Institute and Deputy Director of the Department of Pulmonary Medicine of the Shanghai Chest Hospital, Shanghai, China, provides her expert insight into key data presented at the ESMO 2019 Congress and discusses the latest developments in EGFR TKIs for optimizing outcomes for patients with NSCLC.
Dr Yasushi Goto, Senior Consultant, Division of Thoracic Oncology, National Cancer Center Hospital, Tokyo, Japan, provides his expert insight into key data presented at the ESMO 2019 Congress and discusses the latest developments in EGFR TKIs for optimizing outcomes for patients with NSCLC.
Please Select A Video:
Overview & Learning Objectives
Overview
Stay up to date with the latest developments in the management and treatment of patients with EGFR+ NSCLC with our touchCONGRESS Webinar and Expert Interviews from ESMO Congress 2019 in Barcelona, Spain, 27 September to 1 October 2019.
Prof. Suresh Ramalingam of the Winship Cancer Institute of Emory University reviews the most important emerging data presented at the ESMO Congress 2019 and discusses their potential impact for addressing real-life clinical unmet needs in patients with EGFR+ NSCLC.
Other leading lung cancer experts from Asia, Europe and the United States discuss the latest data presented at the ESMO Congress 2019 in Barcelona and the ESMO Asia Congress in Singapore.
The information in this activity is intended for oncologists and other healthcare professionals involved in the treatment of patients with NSCLC.
Learning Objectives
After watching this touchCONGRESS, you should be able to:
- Recall significant data for third-generation EGFR TKIs in EGFR+ NSCLC
- Recognize differences between the available EGFR TKIs in terms of efficacy and safety
- Describe real-world evidence with third-generation EGFR TKIs and the issues surrounding them

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights