Introduction
With the growing burden of cancer, sustainable solutions are urgently required to ensure access to effective and safe treatments for all patients.1 Health expenditure on cancer in Europe more than doubled between 1995 and 2014, increasing from €35.7 billion to €83.2 billion;2 high-cost cancer drugs are the main drivers of this increase in many countries. Every patient has the right to benefit from medical treatment, regardless of financial means, gender or nationality,3 but at the current rate of spending this will not be achievable in the near future.
A multidisciplinary and multipathway approach facilitated by experts in hospital pharmacy, health economics and oncology is needed. To explore current issues and potential solutions, experts in these fields from across Europe have collaborated on a series of perspectives. The current situation and consequences of reaching the sustainability ceiling (where costs of treatment outweigh the benefits), including definitions of sustainability and value, are discussed further in the first and second articles in this series of three perspectives.4,5
To regulate spending and allow continued – or even improved – access to cancer treatments, changes must be made before the sustainability ceiling is reached.4,5 As detailed in the first two articles of this series, the continuing exponential rise in costs is driven, in part, by the introduction of a large number of new, high-cost treatments. Although innovation and research into new treatments should be encouraged, additional focus is needed on value-based medicine, characterised by evidence-based medicine, patient-centred care and cost-effectiveness.4,6 The availability of additional safe, effective and affordable value-based options will enable health systems to expand access to beneficial treatments for more patients, free up resources for access to other medicines, and relieve pressure on healthcare budgets.7
In this third perspective, we focus on solutions, providing potential options for sustaining cancer care and increasing access to treatments for all patients. In particular, we provide detail on reducing the costs associated with cancer drugs, with a specific focus on the role of biosimilar medicines.
The approach to sustaining cancer care is multifaceted
There are several possible ways to sustain cancer care and aid access to new medicines (Figure 1).
Increased efficiency of healthcare systems
In 2005, the 58th session of the World Health Assembly endorsed a resolution urging member states to work toward sustainable health financing, with a view to achieving ‘universal coverage’ (defined as access to key promotional, preventive, curative and rehabilitative health interventions for all at an affordable cost, thereby achieving equity in access).8
To achieve this goal, the most efficient ways to meet the challenges faced by health systems must be identified and fundamental changes implemented. According to the World Health Organization, all countries can act to improve the efficiency of their health systems, releasing resources that could be used to increase access to treatment, improve or increase service provision, and reduce costs. Unnecessary spending on medicines is listed among the ten leading sources of inefficiency, with key drivers including underuse of generics, as well as higher than necessary prices for medicines. Some medicines are nearly always sold at substantial markups compared with production costs, and prices vary significantly from country to country. These inefficiencies could be addressed by: improving prescribing guidance; requiring, permitting or offering incentives for generic substitution; developing active purchasing-based assessment of costs and benefits of alternatives; ensuring transparency in purchasing and tenders; controlling excessive markups; and monitoring and publicising the prices of medicines.9
Programmes to encourage prevention and early diagnosis
A substantial proportion of cancers are preventable. Primary and secondary prevention strategies have been demonstrated to be cost effective, while also potentially reducing the number of patients with cancer in future generations requiring diagnosis, treatment and follow up. Countries should therefore integrate these measures into existing cancer care plans.10
Earlier diagnosis increases the scope for successful treatment, reducing the burden of disease. While the additional costs of screening and diagnosis may not improve sustainability in the short term, costs would be expected to reduce over time due to reduced morbidity. Furthermore, the added value to the population of life-years gained and cost per life saved suggests that in the longer term earlier diagnosis would be a cost-effective solution and improve value for patients and society.11
Biomarkers to identify responders and nonresponders
Biomarkers of drug response may improve sustainability by enabling increased use of personalised medicine and reducing use of expensive drugs in patients unlikely to benefit owing to underlying disease characteristics. For example, screening for programmed cell death protein 1 (PD1) and/or programmed cell death ligand 1 (PDL1) in immuno-oncology enables targeted treament and reduces use of expensive PD1/PDL1 inhibitors in patients who would not benefit.12 However, impact of biomarker screening may depend on the approval status of a drug in relation to the discovery of the biomarker. If biomarkers are identified before approval, manufacturers may compensate for the potentially reduced market by setting a high price for the drug.13
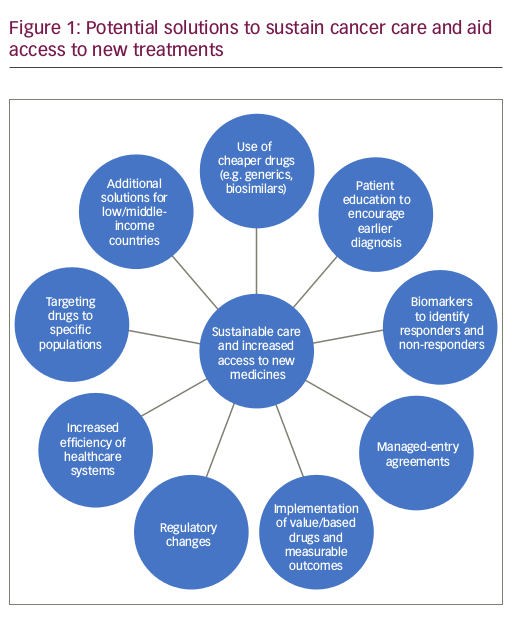
Managed-entry agreements
Managed-entry agreements limit reimbursement of medicines to those subpopulations that are most likely to benefit from treatment. They play a key role in controlling the impact of new medicines on budget, addressing uncertainties about the therapeutic benefit or cost effectiveness of a medicine in a real-world setting, and optimising the use of medicines through patient selection and delivery mechanisms.14 These agreements are increasingly used to obtain lower drug prices in many countries.15 However, there are concerns that this approach may lead to limited transparency because the agreed prices and the nature of the agreements are not usually disclosed to the public.16
Implementation of value-based pricing and measurable outcomes
Innovation in drug development is not always accompanied by financial benefit or a proportionate improvement in important patient outcomes, such as survival. The average launch price of cancer drugs increased by 10% annually between 1995 and 2013,17 yet increased survival benefit only amounted to a few months in some cases. Arguably, current coverage, reimbursement and patent policies do not encourage the development of treatments with clinically meaningful survival benefits. Between 2009 and 2013, only 35% of cancer drugs approved by the European Medicines Agency (EMA) demonstrated prolonged survival and only 10% showed improved quality of life in published evidence. Of those without demonstration of survival benefit at market entry, only 7% and 11% reported extension of life or improved quality of life, respectively, in post-marketing studies.18 New medicines that are approved based on statistically significant data, but that do not demonstrate clinically meaningful benefit, are not of value to society. In fact, they add to the increased absolute costs to society, pushing them ever closer to becoming unaffordable.4
Therefore, prices paid for drugs by payers and patients may not always reflect their true value. Payers are sometimes forced to make coverage decisions based on limited information or where few alternative treatments exist. Different prices may be negotiated between drug companies and various sets of payers, and the price of one product may be determined in conjunction with negotiations over the prices and/or quantities of related products, which would result in prices of several products not reflecting their underlying value.19
Value-based pricing has been proposed as a policy that promotes access while rewarding useful innovation by assigning value to medicines based on a range of clinical and societal parameters.15 This approach could mean that insurers restrict coverage of drugs to those that improve survival time by an economically significant amount.17
Professional organisations, including the European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO), have taken the lead in assessing the real value of treatments by creating value frameworks for drug treatments, which vary in several dimensions.5,20,21 However, there are major limitations to these frameworks, including the lack of a well-defined theoretical basis for determining value and the absence of analyses relating to how payers, physicians or patients would make decisions based on the available metrics.19 One of the challenges in assessing treatment value is how to measure and compare health outcomes and costs. The concept of a threshold value, i.e. how much an individual or society is willing to pay for an incremental health gain, could be used for the valuation of various dimensions of treatment.5,19 However, allocative value (the distribution of assets among population subgroups), technical value (how well assets are used among those in need) and personalised value (how well decisions relate to individuals’ values) should also be considered.22 It is also important to understand that reducing overall costs may sometimes necessitate increasing spending for some services to enable a reduction in others.23
Regulatory changes
Regulatory approval remains a critical step in getting a drug to market, but it does not guarantee market access. Recommendation for reimbursement represents an additional hurdle to market entry in many countries. Pharmaceutical drugs, medical technology or biotechnology products must meet four criteria for reimbursement: quality, efficacy, safety and cost effectiveness.24 This demonstration of clinical and/or monetary benefit is important for market-access schemes aimed at improving access to effective therapies for all patients.
Regulators are not directly involved in determining the price of new medicines; however, they are involved in the ongoing debate over the cost of medicines. Regulatory requirements contribute to high pharmaceutical research and development costs, while regulatory approval in protected markets allows drug manufacturers to charge higher prices. For the sake of affordability, regulators should not yield to pressure to lower standards but should equally be aware of budget pressures from newly-approved drugs.24 Unfortunately, due to the requirements for population selection in preregistration trials, the value of newly-approved drugs is not known at market entry. Standard clinical trials may, therefore, not be best suited for a proper assessment of the societal value of a new medicine.
There are several ways that regulators can contribute to maintaining sustainable expenditure on cancer drugs in Europe. They can encourage clinical trials that measure value and promote better design and more efficient conduct of clinical trials. Health technology assessment (HTA) bodies state that clinical trials supporting marketing authorisation often fail to provide adequate data on quality-of-life or healthcare resource utilisation. To bridge this gap, the EMA and some EU member states have held advice sessions at which regulators, HTA experts and drug developers discussed premarketing clinical trial designs. Regulators in some countries can also facilitate data collection by considering payers’ needs when requesting post-approval studies.24
The role of industry
The pharmaceutical industry can help healthcare professionals (HCPs) and policy makers to understand the differences between medicines and ensure that affordable, value-based medicines are accessible to patients. It has been proposed that the pharmaceutical industry should be rewarded for developing new drugs with better value and governments should only award market exclusivity when genuine innovation has occurred.25 The costs of research and development, marketing, production and distribution, as well as the end prices of new treatments, should be made clear.25 Where there is full transparency, a reasonable and easily understandable price can be set and reimbursed, allowing for price adjustment if market expansion develops differently than expected.
Industry can also be instrumental in generating health economics data for new drugs. Pharmacoeconomic studies should be included from the earliest development phase through to market access and subsequent loss of patent, to enable assessment of value evolution throughout the drug lifecycle;26 these data should be analysed and critiqued by independent health economics investigators. Overall survival and quality-of-life outcomes data should be collected from phase II studies onwards because payers will likely request more robust data to support HTA assessments in the future. More rigorous HTA-based guidance is needed to ensure that the industry is aware of the requirements that must be met for market access and reimbursement. This will allow for earlier planning to ensure all relevant data are collected during development to demonstrate allocative, technical and personalised value, as well as clinical benefit.
Drug pricing based on economic status
Potential solutions for low- and middle-income countries include price discrimination (e.g. through access programmes), compulsory licensing and the differential pricing of drugs based on a country’s specific needs and ability to pay, which may open up opportunities for drug manufacturers to serve these markets while also demonstrating social responsibility.27–29
Use of less expensive medicines
Generic and biosimilar medicines offer the potential to improve value in healthcare7 and may help to combat the substantially increasing costs of cancer drugs, thereby increasing access to cancer treatment.30 These less costly treatment options may give greater autonomy to physicians, enabling them to prescribe the most appropriate treatments for their patients in terms of effectiveness, costs and value.
A generic medicine matches a branded drug in terms of chemical formula, dosage, safety, strength, mode of administration, quality, performance and intended use. Generic medicines are usually chemically synthesised, allowing the existing medicine to be replicated exactly.31 Uptake varies between countries in Europe and is influenced by acceptance, brand loyalty among physicians and patients, pharmacist incentives or automatic substitution, patient cost sharing, physician prescription by international non-proprietary name and price regulation.32,33 For example, France has historically been a brand-loyal market with few mechanisms to drive generic uptake, as demonstrated by the low penetration rate.34 By contrast, Germany encourages generic uptake via prescribing targets, which have driven higher market penetration.35
A biosimilar is a biologic medicine that matches a licensed reference medicine in terms of safety, purity and potency.31,36–39 There is considerable scope for increasing the use of biosimilar medicines;40,41 however, demand-side measures and general policies may be needed to change prescribing habits and realise competition in the market with subsequent cost savings.42
Biosimilar medicines are a promising value-based solution
Improving affordability of cancer care
Biosimilar medicines that have comparable clinical outcomes to reference biologic medicines but at lower cost could enable many more patients with cancer to access effective therapies.43 The availability of biosimilar medicines is expected to improve affordability of cancer care worldwide.44 Evidence shows that the introduction of biosimilar medicines in Europe at reduced prices led to competitive price reductions in reference medicines and beyond.40,45 For example, Norway’s price regulator was offered a discount of 72% for biosimilar infliximab in the country’s 2015 tender for drugs.46 In Germany, three epoetin biosimilar medicines were introduced in 2008 at a cost of 30% below the reference medicine price. A price reduction in the reference medicine was followed by further price reductions of the biosimilar medicines.47
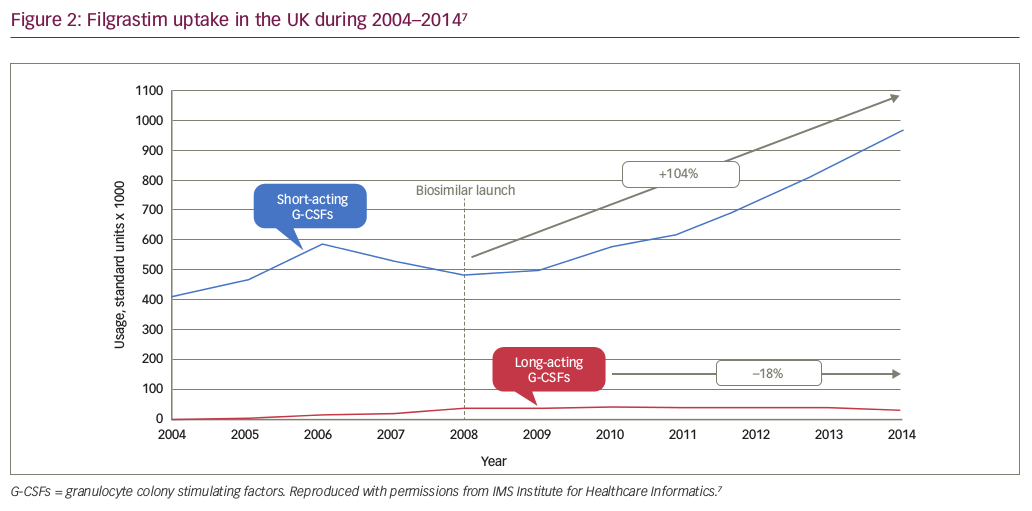
In the UK, Germany and the Netherlands, the availability of a lower cost biosimilar filgrastim (a granulocyte-colony stimulating factor [G-CSF] for treatment of infection and neutropenic fever during chemotherapy) correlated with a 10–20% increase in overall use, mainly occurring earlier in the course of therapy.48 In the UK, guidance for the use of GCSFs was updated after the launch of biosimilar filgrastim to reflect the improved cost effectiveness of the biosimilar versus the reference medicine. As a result, the recommendation for G-CSF initiation was changed from second- to first-line cancer treatment. Uptake of short-acting filgrastim (biosimilar and reference) increased by 104% in the UK between 2008 and 2014 (see Figure 2), representing a significant cohort of patients who may not otherwise have had access to this medicine.7
Potential impact of biosimilar medicines on the price of cancer drugs
The markets best-placed to capitalise on the benefits of biosimilar medicines are those with a functioning competitive environment, where manufacturers are motivated to participate over the longer term and physicians are at the heart of the decision-making process.7 Biosimilar medicines enable stakeholders to benefit from a greater choice of treatments. Competition from biosimilar medicines will contribute to lower drug costs and enable healthcare systems to make significant savings that can be reinvested to tackle the expected increase in cancer prevalence, as well as drive pharmaceutical innovation.35 Savings in the top five European markets could exceed €10 billion between 2016 and 2020, based on direct competition with reference medicines and excluding indirect competition for other in-class or therapy-area-specific product sales.7 By rapidly approving generics and biosimilar medicines, regulators can facilitate competition, driving down prices. A recent information guide for HCPs prepared jointly by the EMA and the European Commission states that biosimilar competition can offer advantages to European healthcare systems as it is expected to improve patient access to safe and effective biological medicines with proven quality.49
Potential barriers to the uptake of biosimilar medicines and suggested solutions
Guidance from the EMA states that 10 years of clinical experience has shown biosimilar medicines approved by the EMA can be used as safely and effectively in all their approved indications as reference biologic medicines.49 Despite this and the potential for substantial cost savings, biosimilar medicines face similar challenges to generics in achieving market penetration.
As biosimilar medicines are still not universally accepted, there is considerable variation in uptake.40,41,50 In 2013, the uptake of biosimilar filgrastim in the five largest European oncology drug markets (France, Germany, Italy, Sweden and the UK) ranged from 35% in France to 91% in Sweden, while uptake of epoetin varied from 16% in France to 53% in Germany.51
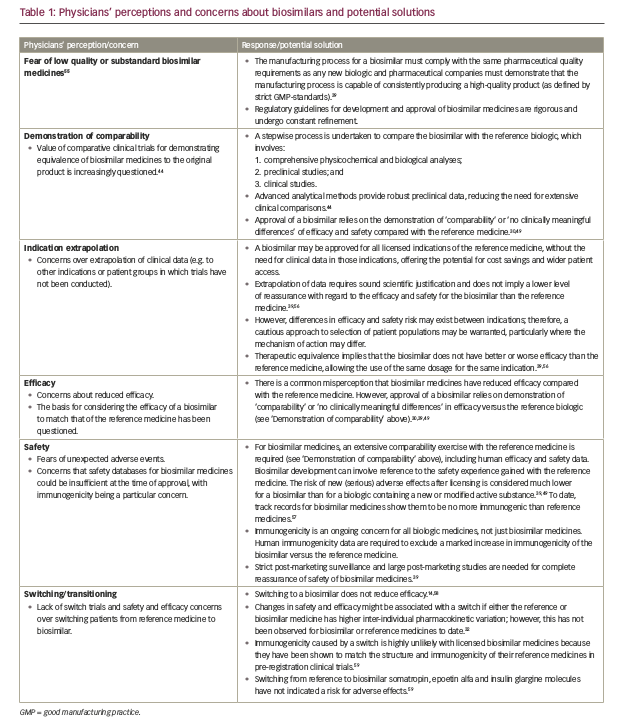
The understanding of biosimilar medicines varies by country and region, and while confidence is growing, concerns remain around the true equivalence of a biosimilar to its reference medicine, as well as immunogenicity, efficacy and safety (Table 1).52 There is often uncertainty about the interchangeability of biosimilar and reference medicines, which is fuelled by variation in definitions and guidance between the EMA and FDA.53 Furthermore, questions have been raised about who will ultimately benefit from associated cost savings. Physicians, patients and payers require balanced education on biosimilar medicines and their role in treatment, while payers need to ensure that physicians and manufacturers are properly incentivised to drive uptake of biosimilar medicines.7,54
Delayed approval and uptake of biosimilar medicines in oncology may have substantial impact on costs and patient access to care. Incentives at a national, regional and local level may be required to promote uptake of biosimilar medicines and utilisation of treatments with the lowest cost.
Conclusions
The biggest threat to sustainability remains continued use of non-value-based therapies. Multidisciplinary, multipathway approaches are needed to achieve sustainable cancer care in Europe. The immediate implementation of initiatives to sustain cancer care should allow continued, and ideally increased, access to effective and safe treatments for all patients. Regulatory bodies, healthcare systems and industry must implement fundamental changes to clarify differences between medicines and ensure that affordable, value-based medicines are available. Furthermore, HCPs should lead the value debate; experts in hospital pharmacy, health economics and medical oncology should collaborate to promote efficiencies and facilitate continued and increased access. Potential savings must be reinvested into improving treatments to ensure patients ultimately benefit, and physicians need to be reassured of this by regulators, payers and industry if they are to lead the value debate.
The development of new cancer treatments that are less costly and offer greater benefit should be encouraged as part of a multipathway plan to secure continued access to effective and safe cancer treatments. Generic and biosimilar medicines represent cost-effective treatment options that may enable payers to give greater autonomy to physicians, providing them with the freedom to prescribe the most appropriate treatments for their patients. Competition from biosimilar medicines is expected to increase affordability and improve access to therapy, enabling the treatment of a greater number of patients. Delayed uptake of biosimilar medicines could have substantial cost and access consequences. Competition lies at the heart of the biosimilar value proposition; however, it must be noted that not all markets have a functioning competitive environment, so are not in a position to benefit from forthcoming biosimilar medicines within oncology. Examining market penetration in individual countries will identify where educational initiatives would be beneficial to allay misperceptions and encourage greater use of generic and biosimilar medicines.
Much work remains to ensure that the sustainability ceiling is not reached and costs of care do not outweigh the benefits. Collaboration among stakeholders in all countries is needed so that sustained access to oncology medicines becomes a reality for all patients within Europe.




