Despite the increasing availability of precision medicine and the recent introduction of immune checkpoint inhibitors in the treatment of many tumours, chemotherapy remains the standard of care for most patients with cancer. Chemotherapy-induced nausea and vomiting (CINV) is highly prevalent among patients with cancer and, without prophylactic therapy, it would occur in up to 80% of patients.1–4 This condition has a substantial impact on the quality of life of patients receiving chemotherapy,5 as well as providing a challenge for healthcare providers; a study from the Acute Oncology Service at University Hospital Southampton Foundation Trust found that 40% of their calls were directly related to CINV.6 Prevention of CINV is essential for the provision of optimal patient-centered cancer care.
Many chemotherapeutic agents have a biphasic pattern of associated CINV symptom development, with an initial peak within 1–2 hours after chemotherapy administration and a second peak at 48–72 hours, which can last for several days after chemotherapy administration. These phases are conventionally defined as acute CINV (≤24 hours after chemotherapy) and delayed CINV (>24–120 hours) (see Figure 1). The prevalence of delayed CINV is around twice that of acute CINV.4 However, patients often are no longer in direct contact with their oncology care team during the delayed phase. This represents a relevant issue in the clinical management of these patients. Furthermore, most antiemetic therapies are more effective for acute than for delayed CINV, presenting a therapeutic challenge.7,8
The risk of developing CINV is strongly related to having CINV in a previous cycle,4 highlighting the importance of proper management of CINV with the first treatment. More than 90% of patients who experience CINV have reported an impact on their daily activities, especially in those who experience delayed CINV.9 Prolonged CINV may lead to severe complications, including dehydration and electrolyte imbalance, potentially causing acute kidney injury or exacerbating chronic renal impairment. These may lead to extended hospitalisation, or increased community visits, with their associated burden on healthcare resources and increased overall cost.10 Antiemetic prophylaxis has been shown to be an economically beneficial strategy for the publicly funded healthcare system.11 The goal of prophylaxis is to reduce the morbidity associated with nausea and vomiting, as well as to preserve quality of life, while maintaining the optimum chemotherapy regimen.12 Recent advances in understanding the pathophysiology of CINV, which involves multiple neurotransmitter and receptor systems, have facilitated the development of new and more effective antiemetic agents.
This review aims to explore the gap between patient and healthcare provider perception of CINV, and factors affecting optimal CINV control, including the patient-related risk factors and the role of healthcare provider adherence to antiemetic guidelines in reducing the residual risk of CINV. The review will discuss the current state of antiemetic regimens, with a focus on international guideline-approved therapies. Finally, we will focus on the role of the recently EU-approved neurokinin-1 receptor antagonist (NK1 RA) rolapitant in this scenario.
The gap between patients’ and healthcare providers’ perceptions of chemotherapy-induced nausea and vomiting
Despite the fact that CINV is one of the most feared adverse events associated with chemotherapy,1,13–15 healthcare providers underestimate the impact of the condition on patients’ daily lives.16 Physicians and nurses typically underestimate the incidence3 and overestimate the control of delayed CINV after highly emetic chemotherapy (HEC).17 Furthermore, patient symptoms are often graded lower by healthcare professionals than by patient self-report.18 Another survey found that physicians and oncology nurses overestimate the incidence of CINV, and underestimate the impact of the condition on patients’ daily lives. These findings were supported by the views of 28% of patients, who felt oncologists underestimated the impact of CINV.16 A study, published in 2015, compared reporting by patients and physicians of six toxicities (anorexia, nausea, vomiting, constipation, diarrhoea and hair loss), based on the information prospectively collected within three randomised trials. Toxicity rates reported by physicians were always lower than those reported by patients.19 Systematic collection of patient-reported outcomes is a valid, reliable, feasible and precise approach to tabulating symptomatic toxicities and facilitates detection of symptoms that are missed by healthcare providers.20 Assessment, communication and education are key to the successful treatment of CINV. A 2008 study found that CINV symptoms were significantly improved by the use of a programme that incorporated patient-reported symptoms using an electronic tool and nursing management guided by evidence-based practice protocols.21
These discrepancies in perception contribute to undertreatment of CINV. Furthermore, inadequate control of emesis may lead to anticipatory CINV during subsequent chemotherapy cycles, which has been reported in up to 25% of patients,22 and arises from a conditioned reflex to stimuli associated with chemotherapy. This usually occurs within 12 hours prior to treatment administration and often increases in frequency and intensity with each subsequent cycle.22,23 The severity of CINV can increase over repeated cycles if antiemetic control is not achieved.24,25 Likewise, the distress associated with CINV can escalate over time26 and potentially lead to discontinuation or suboptimal dose reductions of antitumour therapy.27,28 A 2015 survey reported that 32% of healthcare providers delayed or discontinued a patient’s chemotherapy because of CINV.29 This survey also confirmed that, according to 2,388 healthcare providers who were members of Medscape, an international internet-based continuing medical education provider, delayed CINV presents more difficulties than acute CINV in terms of management.

Risk factors for chemotherapy-induced nausea and vomiting
Established risk-modifying individual factors for CINV include age, specific chemotherapeutic agent used, history of morning sickness and prior emetic episodes within the same or from previous chemotherapy regimens.30–32 Females have a higher incidence of CINV compared with males,30,33 and are, on a group level, less responsive to treatment.34 Patients aged below 65 years are more likely to develop acute CINV than older patients,33 though many older patients experience more delayed CINV than acute.35 Other patient factors associated with increased risk of acute CINV include a history of motion sickness or morning sickness with vomiting; postoperative or radiation-related nausea and vomiting; low alcohol consumption30 and non-smoker status.36 In delayed CINV, only female gender has been significantly associated with failure of antiemetic treatment.36
Treatment factors relate to the type of chemotherapy regimen used. Until 2004, emetogenicity was classified into five levels: minimal (<10%), low (10–30%), low to moderate (30–60%), moderate to high (60–90%) and high (>90%).37 However, in 2004, the Multinational Association for Supportive Care in Cancer™/European Society for Medical Oncology (MASCC/ESMO) and American Society of Clinical Oncology (ASCO) combined the third and fourth categories to give a single group of moderate (30–90%) risk for emesis.38,39 Highly emetogenic chemotherapy (HEC) regimens include cisplatin and cyclophosphamide (≥1,500 mg/m2).6 Moderate emetogenic chemotherapy (MEC) regimens include carboplatin, oxaliplatin, the AC, cyclophosphamide (<1,500 mg/m2), and irinotecan, although AC + cyclophosphamide regimens used in breast cancer are now classified as HEC. Low-risk regimens include the taxanes, mitoxantrone, gemcitabine and 5-fluorouracil.39–41
Carboplatin is commonly used in the treatment of ovarian cancer and of lung cancer. Despite being classified as an MEC by guidelines, it was associated with vomiting rates of over 80% in two studies.42,43 Importantly, carboplatin is also associated with a substantial risk of delayed CINV.44,45 Delayed nausea is also frequently described in patients receiving other moderately emetogenic drugs, such as oxaliplatin46 and irinotecan.47
In a recent study, a model was developed to predict the risk of ≥grade 2 CINV (two or more vomiting episodes or a decrease in oral intake due to nausea) from days 0 to 5 post-chemotherapy using data from 1,198 patients.32 Eight risk factors were identified: age <60 years; the first two cycles of chemotherapy; anticipatory nausea and vomiting; history of morning sickness; hours of sleep the night before chemotherapy; CINV in the prior cycle; patient self-medication with non-prescribed treatments; and the use of platinum or AC-based regimens.
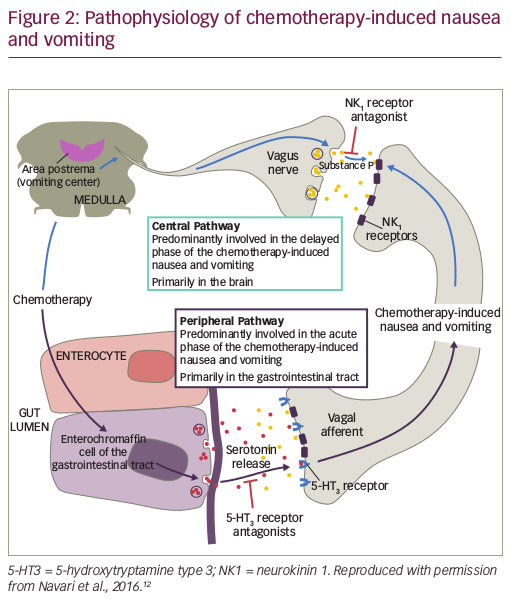
Pathophysiology of chemotherapy-induced nausea and vomiting
CINV is a highly complex condition that involves both the central and the peripheral nervous system (see Figure 2). Chemotherapy causes the release of neurotransmitters in the gastrointestinal tract, cerebral cortex and thalamus, vestibular region and area postrema; these neurotransmitters include dopamine, endorphin, serotonin and the neurokinin receptor ligand substance P.48 The acute phase emesis is largely initiated by serotonin from enterochromaffin cells located in the intestinal mucosa. Serotonin binds to 5-hydroxytryptamine type 3 (5-HT3) receptors located on vagal afferent nerves in the intestinal wall, which send signals via the chemotherapy trigger zone in the area postrema to the vomiting centre in the medulla.49,50 In addition, a central pathway located in the brain is associated primarily with delayed CINV. Chemotherapy triggers the production of substance P, which binds to NK1 receptors in neural networks, mediating the induction of emesis.51 NK1 receptors are also located on vagal afferent terminals in the gastrointestinal tract, suggesting that substance P released from enterochromaffin cells following chemotherapy may also be involved in the acute phase of CINV.51
Blockade of both pathways is needed to optimise CINV control. The peripheral pathway is primarily responsible for acute CINV, while the central pathway controls delayed CINV.49 Antagonists of 5-HT3 and NK1 receptors have therefore been developed as antiemetic agents.
Limitations of current therapeutic options for chemotherapy-induced nausea and vomiting
Therapeutic options for CINV have improved substantially in recent years. The use of corticosteroids for the prophylaxis and management of CINV is well established, even though the mechanism of their action on emesis is poorly understood. Dexamethasone and methylprednisolone are effective as monotherapy52,53 and in combination with other antiemetic agents.53–55 However, corticosteroid use is associated with a number of side effects, which may outweigh its benefits at least in some patients.56 Metoclopramide, lorazepam and other benzodiazepines, and cannabinoids have also been used as antiemetic agents.57
First-generation 5-HT3 RAs such as ondansetron, dolasetron and granisetron, alone and in combination with dexamethasone, have proven to be effective for improving the control of acute CINV with MEC regimens.58 However, they have shown limited effectiveness in controlling delayed CINV.59 Since the introduction of 5-HT3 RAs, acute CINV has become less common, and yet delayed CINV may be underreported and untreated. The second-generation 5-HT3 RA, palonosetron, which has a longer half-life and a higher binding affinity than the first-generation 5-HT3 RAs, has shown better efficacy than first-generation agents in delayed CINV.60,61 However, the latest version of the MASCC guidelines do not recommend the use of palonosetron above other 5-HT RAs.40,62
The addition of a NK1 RA, such as aprepitant,63–65 netupitant66 or fosaprepitant67 to standard 5-HT3 therapy has been shown to decrease CINV over multiple chemotherapy cycles. A combination remedy comprising netupitant and palonosetron has recently received US Food and Drug Administration (FDA) approval after demonstrating efficacy and safety in three randomised controlled clinical trials.66,68,69 However, aprepitant, netupitant and fosaprepitant interact with cytochrome P450 (CYP3A4), which may lead to drug–drug interactions with concomitant medication, an important consideration, particularly in older patients, and may necessitate dose adjustment/reduction of some agents.70–72
Other agents have been investigated recently for CINV control. In a pilot study, the anticonvulsant carbamazepine proved ineffective73 and gabapentin, an anticonvulsant and analgesic, proved no better than placebo in a phase III trial for delayed CINV from HEC.74 However, olanzapine, an atypical antipsychotic agent that blocks multiple neurotransmitters including dopamine, serotonin, catecholamines, acetylcholine and histamine, has demonstrated efficacy in a phase III study in combination with dexamethasone, an NK1 RA (aprepitant or fosaprepitant), and a 5-HT3 RA, in patients with chemotherapy-naïve patients treated with HEC consisting of cisplatin (≥70 mgm2) or cyclophosphamide-doxorubicin based regime. Olanzapine was administered at a dose of 10 mg orally or matching placebo daily on days 1 to 4. Importantly, nausea prevention was the primary endpoint; and complete response (CR) (no emesis and no use of rescue medication) was a secondary endpoint. A total of 380 patients were assessed (192 assigned to olanzapine, and 188 to placebo). The proportion of patients with no nausea was significantly greater with olanzapine than with placebo in the first 24 hours after chemotherapy (74% versus 45%, p=0.002), the delayed phase (25 to 120 hours) after chemotherapy (42% versus 25%, p=0.002), and the overall 120-hour period (37% versus 22%, p=0.002). The CR rate was also significantly better with olanzapine during the three periods: 86% versus 65% (p<0.001), 67% versus 52% (p=0.007), and 64% versus 41% (p<0.001), respectively. Although there were no grade 5 toxic effects reported, some patients receiving olanzapine had increased sedation (severe in 5%) on day 2. The investigators concluded that a 4-drug regime of olanzapine, a 5-HT3 RA, an NK1 RA and a corticosteroid significantly improved nausea prevention, as well as the CR rate, among chemotherapy-naïve patients receiving HEC.75
A recent meta-analysis of 16 studies (15 clinical trials and 1 observational study) demonstrated that the CR rate of olanzapine was superior to other antiemetic regimens, in the delayed and overall phases. Additionally, olanzapine was not superior to standard CINV prophylaxis of the nausea and emesis outcome in the acute phase. Drowsiness and constipation were the most common reported adverse events. Importantly, no grade 3 or 4 adverse events were reported. The authors concluded that olanzapine is effective and safe at reducing CINV during the delayed and overall phase.76 Further investigation of the likely role of olanzapine in antiemetic regimes is therefore warranted.
Effective treatment of nausea in both the acute and delayed phases still remains an unmet clinical need in both patients receiving HEC and those receiving MEC,77 although the addition of olanzapine to standard triple therapy (NK1 RA, 5-HT3 RA and dexamethasone) has shown benefit in patients receiving HEC (cisplatin or cyclophosphamide-doxorubicin–based).75,78,79 Olanzapine is also recommended in the treatment of breakthrough nausea and vomiting. This indication has been incorporated in the recent updated MASCC/ESMO 2016 guidelines.80
Recommendations from international guidelines
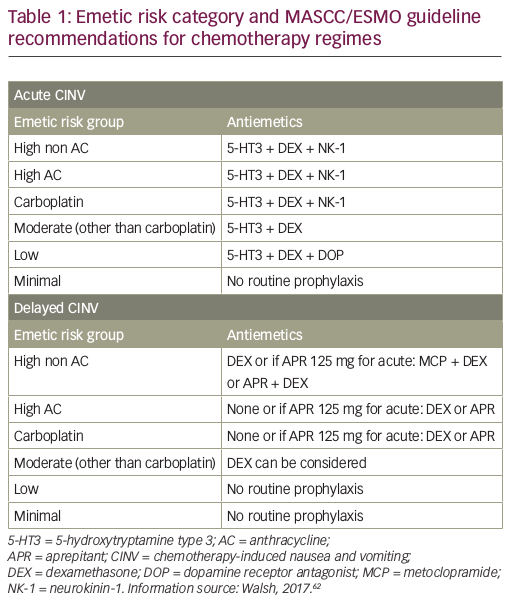
Guidelines for the management of CINV have been published by ASCO, most recently in 2017,81 the National Comprehensive Cancer Network (NCCN; updated annually, last update 2016)41 and MASCC/ESMO (last update in March 2016).40,62 All these guidelines are generally consistent with each other, and recommend scheduled prophylaxis for both acute and delayed CINV associated with HEC and MEC single-day regimens. The MASCC/ESMO antiemetic guidelines are based on the Copenhagen Consensus Conference on Antiemetic Therapy, held in June 2015, and recommend a three-drug regimen including single doses of a 5-HT3 RA, dexamethasone, and an NK1 RA (aprepitant, fosaprepitant, netupitant or rolapitant), given before chemotherapy for the prevention of acute CINV. There are different recommendations for prevention of delayed nausea of high AC, high non AC, carboplatin and other MEC agents (see Table 1).62 Other organisations have also been involved in disseminating guidelines to a wide audience. For example, the Oncology Nursing Society has developed a Putting Evidence into Practice (PEP) book and card for oncology nurses to reference the standard of care for CINV.82 Combinations containing AC have been classified as HEC. The use of AC combination therapy is standard treatment for breast cancer, hence it is very frequently used.
European and US studies have found that patients who had received guideline-consistent antiemetic prophylaxis had significantly better CINV control than those who did not receive guideline-consistent treatment.83–85 However, these studies also reported low adherence to guideline recommendations; this has been confirmed in other analyses.86,87 A recently presented UK study highlighted substantial discrepancies between the antiemetic therapies offered at University Hospital Southampton and the recommended therapies according to MASCC/ESMO, particularly in the delayed phase, where four or five antiemetic drugs were typically prescribed. The authors suggested that it may be more beneficial to prescribe fewer, more effective therapies.6 In addition, an expert European forum of European oncology nurses revealed discrepancies in implementation of antiemetic guidelines in Germany, France, Spain and the UK.88 The forum also revealed variation between the countries in the influence of nurses on treatment decisions involving CINV. Recently, an online survey in France, Germany and Italy found that severe CINV episodes requiring hospitalisation, day hospital or hospitalisation extension were associated with significant expense for health services.89
Rolapitant in the control of chemotherapy-induced nausea and vomiting – discussion and implications for clinical practice with neurokinin receptors
Rolapitant is an orally administered, highly selective, long-acting NK1 RA. It has a high binding affinity towards the NK1 receptor; a positron emission tomography (PET) study found that rolapitant maintained over 90% NK1 receptor occupancy in the cortex up to 5 days following administration of a single 180 mg dose.90 Rolapitant does not interact with cytochrome CYP3A4 and therefore does not require dose adjustments of concomitantly administered drugs metabolised by CYP3A4, particularly dexamethasone.91,92 In contrast, aprepitant is metabolised by CYP3A4, and aprepitant, fosaprepitant and netupitant also both inhibit CYP3A4.92,93 Rolapitant is, however, a moderate inhibitor of CYP2D6, which is involved in the metabolism of other drugs, such as thioridazine, breast cancer resistance protein (BCRP) and P-glycoprotein (P-gp).94 Therefore, caution should be taken when rolapitant is combined with a medicinal product metabolised by CYP2D6, notably those having a narrow therapeutic margin (e.g. propafenone, tamoxifen). Rolapitant has the advantage of a long elimination half-life (approximately 180 hours)95 meaning that a single dose could prevent CINV during the entire at-risk period (0–120 hours), potentially reducing problems of patient adherence.96
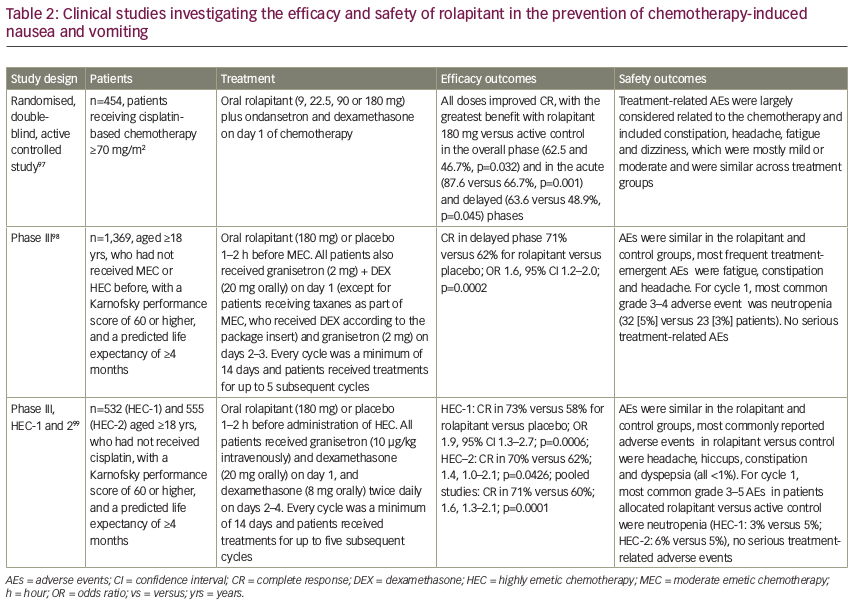
To date, four randomised, double-blind, controlled clinical trials using rolapitant have been performed (see Table 2). In the first, four different doses of rolapitant were evaluated and all were effective and well tolerated, with the greatest benefit observed with rolapitant 180 mg versus active control in the acute (87.6% versus 66.7%, p=0.001) and delayed (63.6% versus 48.9%, p=0.045) phases.97 In another study, rolapitant in combination with granisetron and dexamethasone was well tolerated and demonstrated superiority over active control (5-HT3 RA plus dexamethasone) for the prevention of CINV during the 5-day at-risk period after administration of MEC or regimens containing an AC and cyclophosphamide. While there was no significant difference during the acute phase (83% versus 80%; p=0.14), CR was seen in the delayed phase in 71% versus 62% (p=0.0002) and in the overall phase in 69% versus 58% (p<0.0001) for rolapitant versus active control, respectively. This is important since failure to protect against CINV during the first cycle of chemotherapy is the most significant independent risk factor for delayed CINV during subsequent cycles. In addition, the quality of life score using the Functional Living Index-Emesis (FLIE) was significantly better for patients treated with rolapitant versus the active control (73% versus 67%; p=0.02).98
In two other phase III trials, rolapitant in combination with granisetron and dexamethasone was well tolerated and superior to active control for the prevention of CINV during the at-risk period (120 hours) after administration of highly emetogenic cisplatin-based chemotherapy.99 Rolapitant 180 mg showed the greatest efficacy for CINV prevention in the overall, delayed, and acute phases for patients receiving HEC.97,99 Adverse events in these studies were mostly related to the chemotherapy and included constipation, headache, fatigue and dizziness. These were mostly mild or moderate and similar to the comparative control.
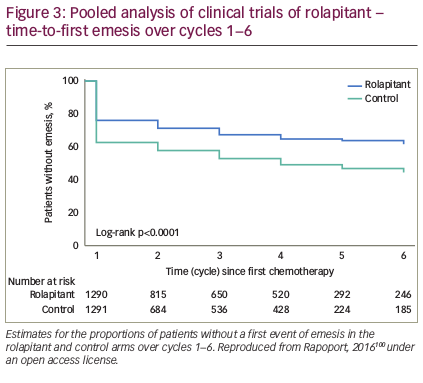
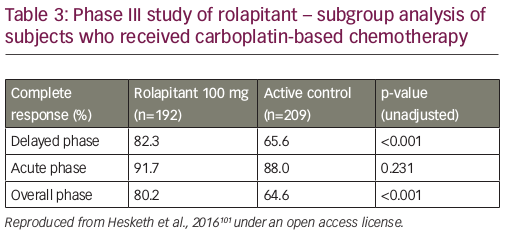
An analysis of pooled data from phase II and III trials concluded that rolapitant was effective and well tolerated over multiple cycles of MEC or HEC with a safety profile that was consistent with that expected for these antiemetic classes and patients receiving chemotherapy (see Figure 3).100 In a recent subgroup analysis, rolapitant 180 mg was superior to placebo in protecting against CINV in patients receiving carboplatin-based chemotherapy; all patients received oral granisetron (2 mg) on days 1 to 3 and oral dexamethasone (20 mg) on day 1 (see Table 3).101 Of note, this subgroup analysis has been recently included in a systematic review and meta-analysis of trials testing the efficacy of NK1 RAs in the prevention of CINV in patients receiving carboplatin-based chemotherapy. Overall, eight trials were included (with aprepitant, fosaprepitant and rolapitant), showing a statistically significant and clinically relevant improvement of CR in all phases (acute, delayed, overall) with triple antiemetic therapy (NK1 RA, dexamethasone and 5-HT3 RA) compared with 5-HT3 RA plus dexamethasone.102 Another subgroup analysis reported that, compared with control, rolapitant improved quality of life in patients receiving MEC or HEC, with significant improvements in FLIE total score (114.5 versus 109.3, p<0.001) compared with control.103
In order to assess possible safety signals in patients taking concomitant drugs that are substrates of CYP2D6 or BCRP, a pooled safety analysis of four double-blind, randomised phase II or III studies was undertaken. Treatment-emergent adverse events were reported in 64% of the rolapitant group and in 65% of the control group. In total, 53% of patients received CYP2D6 substrate drugs, none of which had a narrow therapeutic index and 63% received BCRP substrate drugs. When grouped by concomitant use versus non-use of these drugs, the frequency of treatment-emergent adverse events was similar across both groups, supporting the use of rolapitant with concomitant medications that are substrates of CYP2D6 or BCRP, such as ondansetron, docetaxel or irinotecan.94 Treatment-emergent adverse events related to the use of CYP2D6 or BCRP substrate drugs occurred with similar frequency in the rolapitant and control populations.
Taking into account the different study designs of the currently published phase III studies of aprepitant and roplapitant, there is no significant difference in efficacy among the NK1 RA when used in a triple regimen in HEC and MEC. Rolapitant, however, is used as single dose at the first day of the chemotherapy cycle and has limited CYP3A4-related drug–drug interactions.
As a result of these data, rolapitant has been approved by the FDA for use in combination with other antiemetic agents for the prevention of delayed CINV in adults. It has also been approved by the European Medicines Agency (EMA) in April 2017. The cost of rolapitant is comparable with other NK1 RAs.104 In previous cost–benefit analyses, NK1 RAs have been found to be more effective and less expensive compared to standard care.105
Summary and concluding remarks
Despite the introduction of new targeted agents and immunotherapy, the role of chemotherapy in the treatment of patients with cancer is still prominent. It is therefore important to review antiemetic treatment options and monitor progress to establish the optimal therapy for each patient. Successful CINV control can directly impact outcome of treatment for the patient, adherence to therapy, and whether the patient receives the optimal chemotherapy doses. The introduction of antiemetic agents, such as 5-HT3 and NK1 RAs, and the establishment of evidence-based guidelines, as reviewed in this article, have reduced the incidence of CINV. However, until recently, delayed CINV has remained a challenge, even with these evidence-based guidelines. The introduction of rolapitant represents a further advance in the treatment of delayed CINV. Its single oral administration prior to chemotherapy is convenient and may lead to better control of CINV while on treatment. The lower risk of drug–drug interactions with rolapitant compared with other NK1 RAs might make it beneficial in older people who are often taking multiple medications.
There is a need for further research on the use in antiemetics with emerging anticancer agents, including targeted therapy. Further data are also needed on the utility of rolapitant in the prevention of delayed CINV after high-dose preparative regimens used for autologous and allogeneic haematopoietic stem cell transplants. Aprepitant has proven beneficial in this treatment setting.106
Future clinical trials assessing established and emerging combinations of pharmaceutical agents should include nausea as an endpoint, since the majority of trials are focused on a CR. Tailoring antiemetic treatments to the patient based on individual risk factors will also optimise control of CINV.










