Chemotherapy-induced myelosuppression has long been considered a toxicity that limits the dose of cytotoxic agents, which contributes to significant morbidity and mortality.1 Sub-optimal dose intensity may decrease the efficacy of therapy, thereby compromising progression-free survival (PFS) and overall survival (OS) benefits.2 Chemotherapy-induced myelosuppression is often managed with granulocyte-colony stimulating factors (G-CSFs) and erythropoiesis-stimulating agents (ESAs) in addition to red blood cell (RBC) and platelet transfusions. These supportive care interventions have side effects, risks and limitations. For example, ESAs have been reported to increase cardiac and thromboembolic events in cancer patients.3 A medication, such as trilaciclib given with chemotherapy, that reduces the risk of myelosuppression without major side effects is a useful agent. Trilaciclib, an intravenous (IV) cyclin-dependent kinase (CDK) 4/6 inhibitor, induces a reversible G1 arrest of haematopoietic stem and progenitor cells (HSPCs), leading to the myeloprotection and preservation of immune cells.4
This review outlines the mechanism of action of trilaciclib and highlights the tumour types for which trilaciclib has the potential to reduce myelosuppression without impacting antitumour efficacy. We summarize the clinical data that led to the approval of trilaciclib for extensive-stage small cell lung cancer (ES-SCLC) by the US Food and Drug Administration (FDA) and the promising results from a phase II trial of trilaciclib in combination with chemotherapy in metastatictriple-negative breast cancer (TNBC).
The role of cyclin-dependent kinase 4/6 in the cell cycle
The cell cycle is highly regulated. Often, malignant cells undergo alterations in the cell cycle that lead to uncontrolled cell division. Retinoblastoma (Rb) is a tumour suppressor that plays a key role in cell cycle regulation. CDKs are involved in the phosphorylation of Rb. Once phosphorylated, Rb releases its inhibition of transcription factors. This allows the cell to transition to S phase, which leads to DNA replication. Cancer cells often override this tumour suppressor checkpoint, either by accumulating cyclin D–CDK 4/6 complexes or losing Rb, which leads to unregulated cell replication.5,6 Rb is inactivated in approximately 10% of human cancers, including SCLC, TNBC, urothelial cancer and prostate cancer.7–13 When Rb is inactivated, CDK 4/6 is not required for cell cycle progression.14 As HSPCs and immune cells divide rapidly and are vulnerable to damage during chemotherapy, the transient arrest of the cell cycle by inhibiting CDK 4/6 and, thereby, activating Rb has the potential to protect against myelosuppression and potentially enhance immune activity.15 In tumours with inactivated Rb, the HSPCs and immune cells can be protected without impacting the antitumour effects of chemotherapy. For a more comprehensive review of the molecular underpinnings of CDK 4/6 inhibition, see the recent review from Hu et al.6
The mechanism of action of trilaciclib
Trilaciclib is a potent and selective IV CDK 4/6 inhibitor that blocks the phosphorylation of Rb and induces reversible G1 arrest, thus protecting the Rb competent cells from chemotherapy.4 In preclinical mouse and canine models, trilaciclib was shown to regulate HSPC proliferation in a reversible dose- and time-dependent manner.4 In contrast to currently approved oral CDK 4/6 inhibitors such as palbociclib, ribociclib and abemaciclib, the half-life of trilaciclib is short (the terminal half-life is about 14 hours);16–18 this feature allows it to control tightly the G1 arrest of HSPCs over several chemotherapy half lives. It is thought that trilaciclib may also modulate the immune microenvironment, but the underlying mechanism is unclear.19–21 It has been proposed that trilaciclib enhances antitumour efficacy by reducing the HSPC bias for producing myeloid cells, thereby preserving T-cell production.19–21
Trilaciclib in lung cancer
Rb inactivation is essential for the development of SCLC. As a result, SCLC is highly resistant to CDK 4/6 inhibitors; therefore, the coadministration of CDK 4/6 inhibitors with cytotoxic chemotherapy should not antagonize the efficacy of chemotherapy.4,7 A preclinical study of SCLC in mice further supported this finding by demonstrating that the combination of trilaciclib and topotecan had better tumoricidal efficacy than topotecan alone.4
During dose-finding studies, there was a hypothetical concern that low doses of trilaciclib would produce insufficient G1 arrest, leading to the synchronous release of HSPCs during chemotherapy and increased myelotoxicity. An especially rigorous dose-finding process incorporated pharmacokinetic and pharmacodynamic modelling using animal and human data.20,22 Based on model simulations, escalating doses of up to 192 mg/m2 were tested in healthy volunteers.23 From the pharmacokinetic and safety data, 200 mg/m2 and 240 mg/m2 were tested in the phase Ib dose-finding part of the trial (Phase 1b/2a safety and pharmacokinetic study of G1T28 in patients with ES-SCLC receiving etoposide and carboplatin; ClinicalTrials.gov identifier: NCT02499770), and 240 mg/m2 was the recommended phase II dose.24
Three randomized placebo-controlled phase II trials evaluated trilaciclib in combination with chemotherapy in patients with ES-SCLC.24–26 One of these trials included chemotherapy plus immunotherapy.25 Two of the trials evaluated trilaciclib in patients receiving first-line therapy: one evaluated trilaciclib in combination with etoposide and carboplatin, and the other trial evaluated trilaciclib in combination with etoposide, carboplatin and atezolizumab.24,25 The third trial evaluated trilaciclib in combination with topotecan in patients receiving subsequent-line therapy.26 In all three trials, dose adjustments were allowed for chemotherapy but not for either atezolizumab or trilaciclib. Primary prophylaxis with G-CSF was not permitted in cycle 1, but prophylactic use in subsequent cycles was allowed. G-CSF could be used therapeutically during all cycles. Details of the trials are summarized in Table 1.24–28
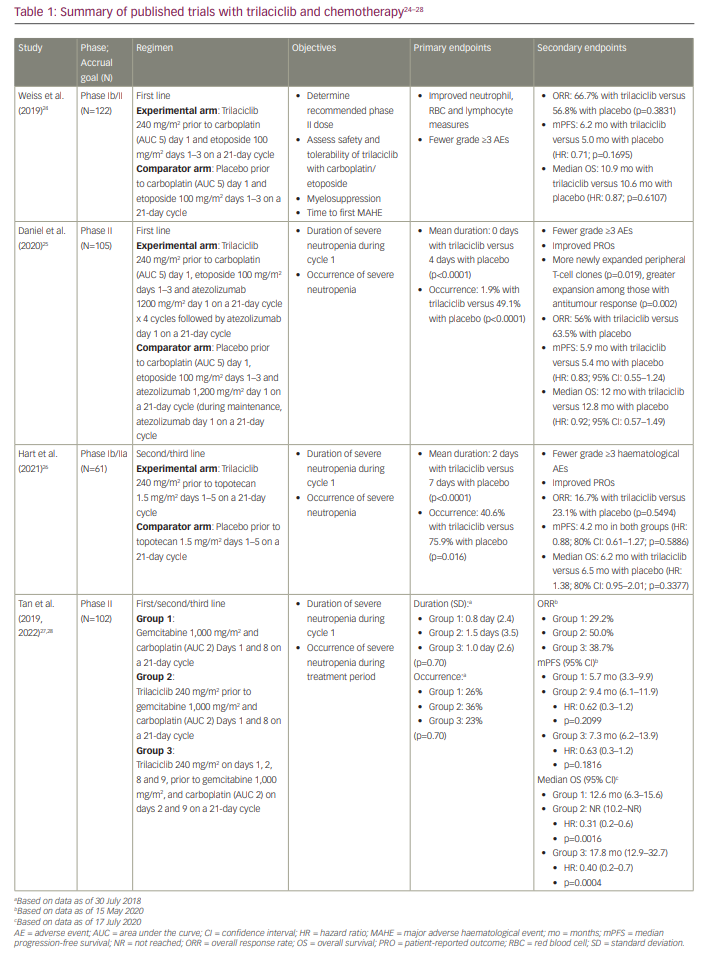
A pooled analysis of these three studies was presented at the Virtual Scientific Program of the 2020 American Society of Clinical Oncology Annual Meeting and later published by Weiss et al.21,29 Of the 242 total randomized patients, 123 received trilaciclib in conjunction with chemotherapy. Patient demographics and disease characteristics were relatively well balanced between the two groups; however, the trilaciclib group had more men (72.4%) and current smokers (39.8%). The primary endpoint was severe neutropenia in cycle 1, defined as an absolute neutrophil count of <0.5 x 109 cells/L, which occurred in 14 patients (11.4%) in the trilaciclib group and 63 patients (52.9%) in the placebo group (p<0.0001). The duration of severe neutropenia in cycle 1 was a mean of 0 days (standard deviation [SD]: 1.8) with trilaciclib versus 4 days (SD: 5.1) with placebo (p<0.0001). Significantly fewer patients in the trilaciclib arm required G-CSF administration, RBC transfusion and ESA administration. In addition, fewer patients experienced grade 3/4 anaemia or thrombocytopenia. G-CSF was administered less frequently in the trilaciclib arm than the placebo arm (28.5% versus 56.3%; p<0.0001). Fewer patients in the trilaciclib arm than the placebo arm had serious infectious adverse effects (AEs) (6.5% versus 10.1%) and received IV antibiotics (19.5% versus 23.5%). The median duration of treatment was four cycles in all treatment arms. Patients in the trilaciclib arms required fewer dose reductions of chemotherapy than those in the placebo arms, with a dose reduction incidence of 2.8 per 100 cycles for trilaciclib and 9.3 for placebo (p<0.0001).
Antitumour efficacy was similar among those who received trilaciclib and those who did not. There were comparable PFS and median OS in both arms. Objective response was achieved in 49.1% of evaluable patients receiving trilaciclib (56 of 114) and in 51.8% of those receiving placebo (59 of 114). The median duration of response was 5.7 months (95% confidence interval [CI]: 4.7–7.0) and 4.6 months (95% CI: 4.1–5.0) for the trilaciclib and the placebo group, respectively. The median PFS was 5.3 months in the trilaciclib arm and 5.0 months in the placebo arm (hazard ratio [HR]: 0.80; 95% CI: 0.61–1.06; p=0.1404). The median OS was 10.6 months in both arms (HR: 1.00; 95% CI: 0.75–1.35; p=0.8136).
The addition of trilaciclib did not increase toxicity. Fatigue and nausea were the only AEs attributable to trilaciclib that occurred in more than 10% of patients. Trilaciclib AEs included injection site reactions (13.9% with trilaciclib versus 2.5% with placebo), phlebitis/thrombophlebitis (9.0% versus 0.8%) and acute drug hypersensitivity reactions (4.1% versus 3.4%). Grade 3 interstitial lung disease/pneumonitis occurred in one patient receiving trilaciclib 2 months after discontinuation and while receiving a confounding medication.30 In August 2019, trilaciclib was granted the Breakthrough Therapy designation.31 On 12 February 2021, it received FDA approval for use in adults with ES-SCLC prior to the administration of a platinum/etoposide- or topotecan-containing regimen to decrease the incidence of chemotherapy-induced myelosuppression.32
In a retrospective pooled analysis by Ferrarotto et al., the need for supportive interventions was significantly reduced in patients receiving trilaciclib.33 The use of G-CSF, ESAs and RBC transfusions on or after week 5 was significantly lower in patients receiving trilaciclib than in those receiving placebo (G-CSF: 28.5% versus 56.3%, p<0.0001; ESAs: 3.3 versus 11.8%, p=0.0254; RBC transfusions: 14.6% versus 26.1%, p=0.0252). Trilaciclib significantly reduced the duration of severe neutropenia, irrespective of G-CSF administration.33
As part of the three randomized placebo-controlled phase II trials that evaluated trilaciclib in combination with chemotherapy in patients with ES-SCLC, patient-reported outcomes were also investigated.24–26 Measures included the Functional Assessment of Cancer Therapy (FACT)-Anemia, FACT-Lung and FACT-General.34–36 At baseline, mean quality-of-life scores from the FACT tools were similar. For each of the patient-reported outcome endpoints, patients who received trilaciclib were found to have a significantly longer median time to confirmed deterioration than those who received placebo.29
To evaluate the cumulative myeloprotective effects of trilaciclib, Dómine Gómez et al. prospectively defined an exploratory composite endpoint that consisted of five major adverse haematological events (MAHE): all-cause hospitalizations, all-cause chemotherapy dose reductions, febrile neutropenia, prolonged severe neutropenia, and RBC transfusions on or after week 5.37 The authors then evaluated this endpoint for the three trilaciclib phase II trials in ES-SCLC. In both the individual trials and in the pooled analysis, trilaciclib significantly reduced MAHE. No significant difference in the rate of all-cause hospitalizations was found between trilaciclib and placebo; however, trilaciclib did significantly extend the amount of time patients remained free of MAHE compared with placebo (not estimable versus 4.1 weeks; HR: 0.41; 95% CI: 0.29–0.60; p<0.0001).
To characterize the immune effects of trilaciclib further, Stevens et al. evaluated T-cell activation in patients who received trilaciclib as first-line treatment for ES-SCLC as part of two of the three lung cancer clinical trials.38 In one trial, patients received a combination of carboplatin and etoposide with either trilaciclib or placebo;24 in the other trial, patients received etoposide, cisplatin and atezolizumab with either trilaciclib or placebo.25 The ImmunoSEQ Assay (Adaptive Biotechnologies, Seattle, WA, USA) was performed on genomic DNA from peripheral blood mononuclear cells and archival tumour tissue. The complementarity determining region 3 (CDR3) regions of T-cell receptor (TCR) beta were amplified and sequenced to identify and quantitate the abundance of each unique TCR beta CDR3.38 Clonal frequencies were compared at baseline and on treatment. In both trials, peripheral T-cell clonal expansion was greater among patients receiving trilaciclib versus placebo. Comparing patients who responded to treatment, patients who received the combination of trilaciclib, carboplatin and etoposide had more peripheral clonal expansion (median 23 versus 12 clones; p=0.04) and a greater number of tumour-associated expanded clones than those who received placebo (6 versus 1.5 clones; p=0.04). Similarly, patients who received etoposide, cisplatin, atezolizumab, and trilaciclib and responded to treatment were also found to have significantly more peripheral clonal expansion (median 90 versus 43 clones; p=0.002) and more newly expanded peripheral T-cell clones than responders receiving placebo (68 versus 11 clones; p=0.003). This suggests that, for patients treated with trilaciclib plus either chemotherapy or chemoimmunotherapy, increased T-cell clonal expansion is associated with clinical response and that trilaciclib may enhance the antitumour immune response in patients with ES-SCLC.
A number of studies suggest that health-related quality of life is worse for patients with lung cancer than for those with other malignancies.39–41 Therefore, improving the quality of life for these patients is imperative. The studies described above demonstrate the myelopreservative effects associated with trilaciclib and improved quality of life in patients with ES-SCLC who receive trilaciclib during chemotherapy treatment.24–26 Patients receiving trilaciclib had improved laboratory values and fewer incidences of febrile neutropenia and hospitalizations from chemotherapy-induced myelosuppression or sepsis. Given the aggressive nature of the disease and the often high-symptom burden experienced with ES-SCLC, improving treatment tolerability has the potential to greatly improve the quality of life on treatment. Fewer interventions (e.g. G-CSF, ESAs and RBC transfusions) and fewer infections and hospitalizations related to neutropenia mean that patients spend less time within the healthcare system and more time on activities important to them. In addition, correlative studies suggest that trilaciclib use for ES-SCLC in combination with chemotherapy and chemoimmunotherapy may enhance the antitumour immune response.38 Large prospective correlative studies are needed to determine whether the clinical benefits of trilaciclib observed to date, such as an increased relative dose intensity of chemotherapy received and the enhanced antitumour immune response, translate to meaningful PFS or OS benefit.
Trilaciclib in breast cancer
Chemotherapy is an essential and active component of treatment for metastatic TNBC. More recently, immune checkpoint inhibitors have been approved. Pembrolizumab, a programmed cell death protein 1 (PD-1) inhibitor, has been approved in combination with chemotherapy for patients with advanced or metastatic TNBC whose tumours have a programmed cell death protein ligand 1 (PD-L1) combined positive score of ≥10.42 Other approved targeted therapies include poly adenosine diphosphate-ribose polymerase inhibitors, such as olaparib and talazoparib, for patients harbouring BRCA mutations and the antibody–drug conjugate sacituzumab govitecan-hziy for patients with metastatic TNBC who have received two or more prior systemic therapies.43–45 For metastatic TNBC that is PD-L1-negative or with BRCA wild-type, chemotherapy is still a mainstay of therapy. Gemcitabine and carboplatin chemotherapy treatment gives rise to treatment-limiting cumulative myelosuppression that can potentially compromise antitumour efficacy. Additionally, chemotherapy-induced immune cell toxicity may limit the immune response against TNBC.46 It is hypothesized that trilaciclib infusion before chemotherapy treatment can protect bone marrow from chemotherapy-induced toxicity and enhance immune response in patients with TNBC.4,19,20,47
A randomized multicentre phase II trial (Phase 2 study of the safety, efficacy, and pharmacokinetics of G1T28 in patients with metastatic triple negative breast cancer receiving gemcitabine and carboplatin chemotherapy; ClinicalTrials.gov identifier: NCT02978716) was conducted to evaluate the myeloprotective effects of trilaciclib in 102 patients with metastatic TNBC who had received no more than two previous lines of chemotherapy.27 On 21-day cycles, group 1 received gemcitabine 1,000 mg/m2 and carboplatin (chemotherapy) on days 1 and 8, group 2 received trilaciclib 240 mg/m2 before gemcitabine and carboplatin treatment on days 1 and 8, and group 3 received trilaciclib on days 1, 2, 8 and 9 before gemcitabine and carboplatin treatment on days 2 and 9. The primary endpoints were the duration of grade 4 neutropenia in cycle 1 and the occurrence of grade 4 neutropenia throughout treatment. Key secondary endpoints included RBC transfusions on or after week 5, the administration of G-CSF, platelet transfusions and antitumour efficacy (objective response rates [ORR], PFS and OS).
The treatment groups had similar baseline tumour characteristics and demographics. Overall, 25% of patients had liver metastases, and 63% received study therapy as first-line treatment for metastatic disease. Adding trilaciclib to chemotherapy did not significantly improve either the primary myelosuppression endpoints or the duration/occurrence of severe neutropenia. Those on study therapy received fewer RBC transfusions on and after week 5. Overall, trilaciclib did not increase toxicity. Trilaciclib increased the duration of exposure to gemcitabine plus carboplatin and increased the number of cycles received from four with gemcitabine and carboplatin alone to seven or eight.
Despite a longer duration of treatment with gemcitabine and carboplatin in patients who received trilaciclib, grade 3/4 haematological toxicities were similar across groups. No serious AEs or AEs leading to treatment discontinuation were related to trilaciclib; trilaciclib-related toxicities included low-grade headache, injection site reactions and local phlebitis. The most common AEs were anaemia (73%), neutropenia (70%) and thrombocytopenia (60%) in group 1; neutropenia (82%), thrombocytopenia (55%) and anaemia (52%) in group 2; and neutropenia (66%), thrombocytopenia (63%) and nausea (49%) in group 3. One patient in group 1 and one patient in group 2 developed febrile neutropenia. Serious treatment-emergent AEs were reported in 10 patients (33%) in group 1, 11 (33%) in group 2 and 4 (11%) in group 3.
Of the patients who were evaluable for response, objective response was seen in 33% (8 of 24; 95% CI: 15.6–55.3) of patients in group 1, 50% (15 of 30; 95% CI: 31.3–68.7; p=0.23) in group 2 and 37% (11 of 30; 95% CI: 19.9–56.1; p=0.68) in group 3. No significant difference in PFS was seen with the addition of trilaciclib to gemcitabine plus carboplatin (chemotherapy). The median PFS was 5.7 months in patients treated with gemcitabine plus carboplatin (group 1), 9.4 months in patients given trilaciclib on days 1 and 8 (group 2) and 7.3 months in patients given trilaciclib on days 1, 2, 8 and 9 (group 3). The HRs of 0.59 and 0.60 comparing chemotherapy with chemotherapy plus trilaciclib, respectively, were not significant. Improvement in OS was observed in patients receiving chemotherapy and trilaciclib versus chemotherapy alone, which was statistically significant: 20.1 months (interquartile range [IQR] 9.4–not reached [NR]) in group 2 and 17.8 months (IQR: 8.8–NR) in group 3 versus 12.6 months (IQR: 5.8–15.6) in group 1 (group 3 versus group 1 two-sided p=0.0023).
The final antitumour efficacy results from this phase II study (ClinicalTrials.gov identifier: NCT02978716) were presented at the San Antonio Breast Cancer Symposium of 2020 and published in 2021.28,48 The results are summarized in Table 1. Of the 102 eligible patients, median follow-up was 8.4 months (range: 0.1–25.7) for group 1, 14.0 months (range: 1.3–33.6) for group 2, and 15.3 months (range: 3.5–33.7) for group 3. Patients receiving trilaciclib prior to chemotherapy had a higher ORR and longer PFS and OS than those who received chemotherapy alone: ORR in group 1 was 29.2% versus 44.3% in groups 2 and 3; the median PFS in group 1 was 5.7 months (95% CI: 3.3–9.9) versus 9.0 months (95% CI: 6.4–11.3) in groups 2 and 3 (HR: 0.62; 95% CI: 0.4–1.1; p=0.1291); the median OS was 12.6 months (95% CI: 6.3–15.6) in group 1 versus 19.8 months (95% CI: 14.0–NR) in groups 2 and 3 (HR: 0.37; 95% CI: 0.2–0.6; p<0.0001).
Outcomes according to CDK 4/6 status, immune subtyping and TCR status were also reported. Outcomes were similar in tumours that were CDK 4/6 dependent, independent, or indeterminate. Trilaciclib did not impact the efficacy of chemotherapy in those who had CDK 4/6-dependent or -variable tumours. No significant difference in outcomes was seen in patients with known CDK 4/6-independent or -variable tumours. In terms of immune subtyping, the expression of PD-L1, assessed using the Ventana SP-142 PD-L1 Assay (Ventana Medical Systems, Inc., Oro Valley, AZ, USA),49 was positive (defined as ≥1% of the total tumour area containing PD-L1–labelled immune cells) in 55.2% of those receiving trilaciclib and 63.0% of those in the chemotherapy only group. Trilaciclib given prior to chemotherapy improved PFS and OS irrespective of PD-L1 status; however, a larger survival benefit was observed for the PD-L1-positive population. In the PD-L1-positive population, OS was 10.5 months (95% CI: 6.3–18.8) in group 1 versus 32.7 months (95% CI: 17.7–NR; HR: 0.34 [95% CI: 0.2–0.7]) in groups 2 and 3. In the PD-L1-negative population, OS was 13.9 months (95% CI: 12.6–NR) in group 1 versus 17.8 months (95% CI: 13.1–NR; HR: 0.48 [95% CI: 0.2–1.2]) in groups 2 and 3. Similar results were observed when the total population was analysed by immune signature, interferon gamma signature, expanded interferon gamma signature and six-class immune signature.
To assess the effect of trilaciclib on the peripheral T-cell repertoire, TCR immunosequencing was performed. Simpson clonality, a measure of how much of the T-cell repertoire is composed of expanded clones, was used.50,51 A significant decrease in Simpson clonality was observed over time in patients who received trilaciclib with chemotherapy compared with those who received chemotherapy alone (p=0.012). When patients were stratified above or below the median Simpson clonality, a trend towards improved OS was observed for those with lower peripheral clonality, with a statistically significant improvement observed for those who received trilaciclib (p=0.02). Responders receiving trilaciclib with chemotherapy had more newly detected clones than those in the chemotherapy alone group (p=0.09). Among responders, a trend towards improved survival and a higher fraction of newly detected expanded clones was observed for those who received trilaciclib. These correlative analyses demonstrate that trilaciclib given in combination with chemotherapy benefits patients regardless of CDK 4/6 status and PD-L1 expression. TCR testing and immune subtyping data suggest that trilaciclib in combination with chemotherapy not only preserves but also enhances immune system function, potentially explaining the antitumour activity observed in patients receiving treatment with trilaciclib, gemcitabine and carboplatin. Given the small number of patients in each subgroup, these findings are considered hypothesis generating.
The current data suggest that trilaciclib may have differential effects depending on tumour type and chemotherapy regimen.19,47 Trilaciclib may have a predominant antitumour activity in some situations and more myeloprotective effects in others. As trilaciclib antitumour activity is suggested to be immune mediated, the type of chemotherapy and tumour type being treated are likely important considerations when anticipating differential outcomes. Trilaciclib may support more antitumour activity in tumour microenvironments favourable to immune activation and with therapy that enhances immune-mediated cytotoxicity. Trilaciclib has been shown to enhance antitumour immune response through the direct stimulation of T-cell and suppression of regulatory T-cell growth.52,53 When given before chemotherapy infusions, trilaciclib may prevent chemotherapy-induced T-cell apoptosis and bone marrow myeloid skewing and preserve intratumoural cytotoxic T-cell proliferation.20,53,54 As trilaciclib enhances immune activation through the differential arrest of cytotoxic and regulatory T-cell subsets followed by the prompt recovery of cytotoxic T-lymphocytes over regulatory T-cells in tumours, the enhancement of cytotoxic T-cells may augment the antitumour effect and, thereby, increase survival.15 In addition to the favourable effects of trilaciclib on the tumour immune microenvironment, patients receiving trilaciclib were able to receive chemotherapy for longer, which may have added to the favourable effect on OS.
The gain in OS seen with the chemotherapy plus trilaciclib in metastatic TNBC in this small phase II study (ClinicalTrials.gov identifier: NCT02978716) has prompted a phase III randomized double-blind placebo-controlled study (A phase 3, randomized, double-blind study of trilaciclib or placebo in patients receiving first- or second-line gemcitabine and carboplatin chemotherapy for locally advanced unresectable or metastatic triple-negative breast cancer [PRESERVE 2]; ClinicalTrials.gov identifier: NCT04799249) investigating trilaciclib versus placebo in combination with first- or second-line gemcitabine and carboplatin (chemotherapy) in patients with locally advanced unresectable or metastatic TNBC. The primary endpoint is OS, and the study will have two cohorts. In Cohort 1, trilaciclib will be evaluated in the first-line setting, regardless of PD-L1 status, in patients who are PD-1 and/or PD-L1 inhibitor therapy naïve.55 In Cohort 2, trilaciclib will be evaluated in the second-line setting following prior therapy with a PD-1/PD-L1 inhibitor. Additional details of this trial and other ongoing trials are summarized in Table 2.55–59
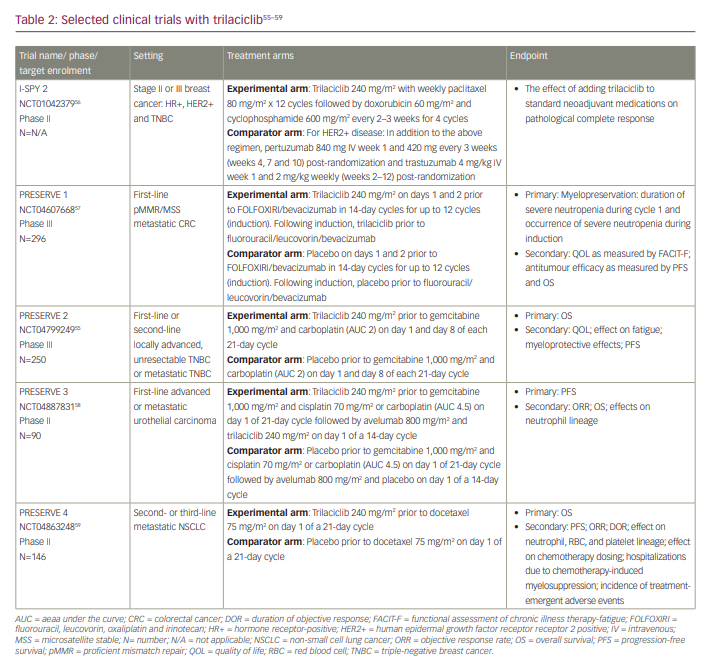
The potential for trilaciclib to improve outcomes in other breast cancer subtypes is being evaluated in the phase II randomized neoadjuvant breast cancer I-SPY 2 Trial (Investigation of serial studies to predict your therapeutic response with imaging and molecular analysis 2; ClinicalTrials.gov identifier: NCT01042379).56 This trial is enrolling for all early-stage breast cancer subtypes, including hormone receptor-positive human epidermal growth factor receptor 2 (HER2)-positive and TNBC. All patients will receive standard neoadjuvant treatment, including chemotherapy and anti-HER2 therapy for HER2-positive disease, with patients in two study arms also receiving an anti-PD-1 checkpoint inhibitor plus paclitaxel (chemotherapy) before surgery. The impact of trilaciclib on tumour response, tumour immune microenvironment and myelopreservation will be evaluated.
Trilaciclib in other cancers
Given the promising results seen for ES-SCLC and TNBC, the myelopreservative and potential enhancement of the antitumour immune effects of trilaciclib in combination with chemotherapy and immunotherapy are being investigated in other solid tumours, including metastatic colorectal cancer, metastatic urothelial carcinoma and metastatic non-small cell lung cancer in a series of trials. An ongoing phase III double-blind placebo-controlled trial (PRESERVE 1: A phase 3 randomized, double-blind trial of trilaciclib versus placebo in patients receiving FOLFOXIRI/bevacizumab for metastatic colorectal cancer; ClinicalTrials.gov identifier: NCT04607668) is randomizing patients with metastatic proficient mismatch repair or microsatellite stable colorectal cancer to trilaciclib versus placebo prior to treatment with FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin and irinotecan) and bevacizumab every 14 days.57 Its primary endpoint is myelosuppression, and secondary endpoints include PFS, OS and quality-of-life measures. PRESERVE 3 (A phase 2, randomized, open-label study of trilaciclib administered with first-line platinum-based chemotherapy and avelumab maintenance therapy in patients with untreated, locally advanced or metastatic urothelial carcinoma [PRESERVE 3]; ClinicalTrials.gov identifier: NCT04887831) is a randomized open label phase II trial evaluating trilaciclib in combination with anti-PD-L1 therapy in first-line metastatic urothelial carcinoma.58 Arm A will receive trilaciclib with gemcitabine/platinum chemotherapy in 21-day cycles followed by trilaciclib and maintenance avelumab every 14 days. Arm B will receive gemcitabine/platinum chemotherapy followed by maintenance avelumab every 14 days. The primary endpoint is PFS, and the secondary endpoints include ORR, OS and effects on the neutrophil lineage. PRESERVE 4 (A phase 2 randomized, double-blind, clinical trial of trilaciclib versus placebo in patients with metastatic non-small cell lung cancer [NSCLC] treated with docetaxel in the 2nd/3rd line setting [PRESERVE 4]; ClinicalTrials.gov identifier: NCT04863248) is a phase II randomized, double-blind trial evaluating trilaciclib or placebo in combination with docetaxel for patients with metastatic non-small cell lung cancer in the second- or third-line setting.59 The primary endpoint is OS, and secondary endpoints include PFS, ORR, duration of response, effect on neutrophil, RBC, platelet lineage, effect on chemotherapy dosing, hospitalizations due to chemotherapy-induced myelosuppression and incidence of treatment-emergent AEs.
Conclusions
To date, clinical trials of patients with ES-SCLC have demonstrated that trilaciclib has a myelopreservative effect when given prior to chemotherapy in first- and subsequent-line therapy. Results of these trials have led to the FDA approval of trilaciclib in these settings. In a phase II trial of patients with metastatic TNBC, trilaciclib given prior to chemotherapy improved OS.27,28,48 As this was a relatively small study and OS was a secondary endpoint, this is being further evaluated in the phase III PRESERVE 2 trial.55 The other PRESERVE studies (1, 3 and 4) are evaluating trilaciclib in combination with chemotherapy (one with chemoimmunotherapy) in metastatic colorectal cancer, metastatic urothelial carcinoma and metastatic non-small cell lung cancer, respectively.57–59 The immune effects of trilaciclib in the peripheral blood preliminarily show that the addition of trilaciclib prior to chemotherapy results in enhanced T-cell activation. Currently enrolling trials include laboratory investigations of the potential immune-based mechanisms of trilaciclib, which will contribute to further understanding of its role in the treatment of solid tumours.










