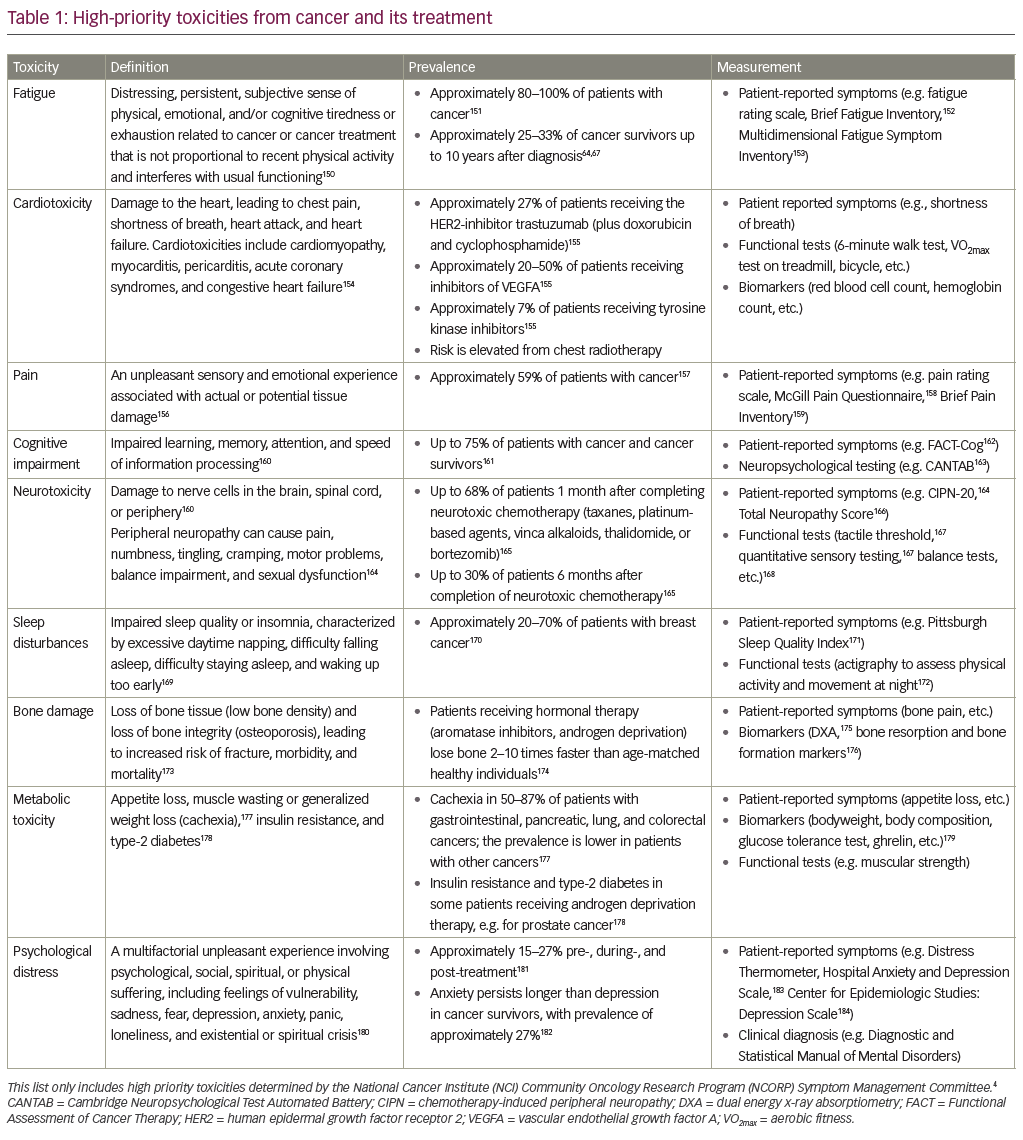
Forty percent of Americans will be diagnosed with cancer in their lifetimes.1 Due to continued advances in cancer detection and treatment, there are an unprecedented number of cancer survivors. In fact, the 5-year survival rate for all cancers has increased from 50% in the 1970s to 67% during the period of 2006–2012.2 The two most prevalent types of cancer, prostate and breast cancer, have reached a 5-year survival of 99% and 89%, respectively.3 Despite advances in cancer treatments that have improved overall survival, patients still face toxicities from cancer and its treatment that impair quality of life, productivity, and sometimes prohibit the use of maximally effective treatments. The National Cancer Institute (NCI) Community Oncology Research Program (NCORP) Symptom Management Committee has identified high-priority cancer- and treatment-related toxicities as areas for future research. These toxicities are divided into first and second tiers based on their potential to rapidly, significantly, and positively affect cancer care, if they are alleviated.4 The first tier of toxicities includes fatigue, cardiotoxicity, pain, cognitive impairment, and neurotoxicity; the second tier of toxicities includes sleep disturbances, bone damage, metabolic toxicity, and psychological distress. Table 1 describes each toxicity, its prevalence, and how it can be studied using patient report, clinical assessments, and objective biomarkers. We focused our review on the highest-priority toxicities identified by the NCORP Symptom Management Committee;4 and though we acknowledge the importance of other toxicities (nausea, vomiting, sexual dysfunction, hair loss, mucositis, lymphedema, etc.), they are beyond the scope of this review.
A growing body of literature suggests that exercise is safe, feasible, and effective in alleviating or preventing toxicities from cancer and its treatment for patients across the treatment continuum.5–7 As we review herein, dozens of randomized controlled trials (RCTs) have assessed how toxicities from cancer and its treatment are affected by a wide range of exercises, including aerobic exercises such as walking, running, and stationary cycling; resistance exercises such as supervised weight training and the use of therapeutic resistance bands; and other forms of exercise such as yoga and Tai Chi Chuan (herein referred to as Tai Chi). Exercise is effective in treating a wide range of toxicities likely due to its established beneficial effects on multiple biological pathways (e.g. inflammation,8,9 endocrine hormones,9–11 the hypothalamic-pituitary-adrenal [HPA] axis, 9,12 and mitochondria13), psychological pathways (e.g. improved self-worth, improved self-esteem from mastering new skills, greater sense of control, time away from stress),14 and social pathways (e.g. more positive social interactions via improved self-confidence, socializing during/after exercise).15 Section 5 reviews biological mechanisms of the toxicities and effects of exercise in more detail. Indeed, exercise can complement pharmacological therapies, which most often are selected or designed to treat a single toxicity or pathway. Exercise is also feasible because it can be designed to accommodate the unique needs of each patient based on age, health status, and ability.6
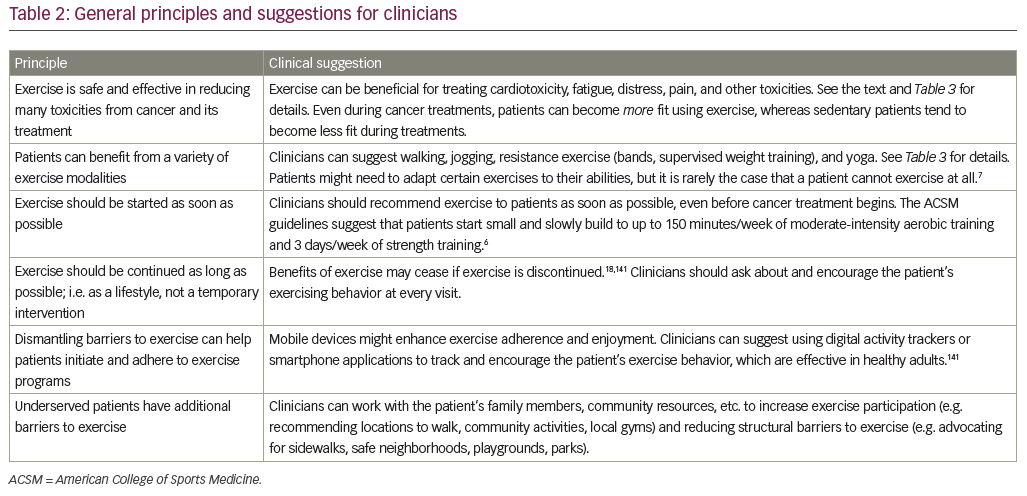
The goal of this paper is to present a broad narrative review of the use of exercise for toxicity management in patients with cancer. First, we review studies of exercise before cancer treatment, during cancer treatment, and after completion of cancer treatment. Next, we review biological mechanisms by which exercise exerts its beneficial effects, to better understand how it can be optimized and individualized. We review disparities in populations who have been understudied with respect to exercise during cancer as well as barriers preventing patients from exercising. Finally, we review technology to track and facilitate exercise adherence. We conclude with suggestions for clinicians to help patients select and adhere to an exercise program. This growing body of literature provides best practice suggestions in most cases, but has not yet reached a level of evidence required to produce definitive guidelines. Our major conclusions and clinical suggestions are summarized in Table 2, with more detailed suggestions for key toxicities listed in Table 3. Our review of studies is not complete, but rather highlights RCTs of exercise, examining one of NCORP’s high-priority toxicities for studies that are particularly definitive (i.e. large sample size, rigorous study design) or particularly informative (i.e. novel measures or interventions).
Exercise before cancer treatment
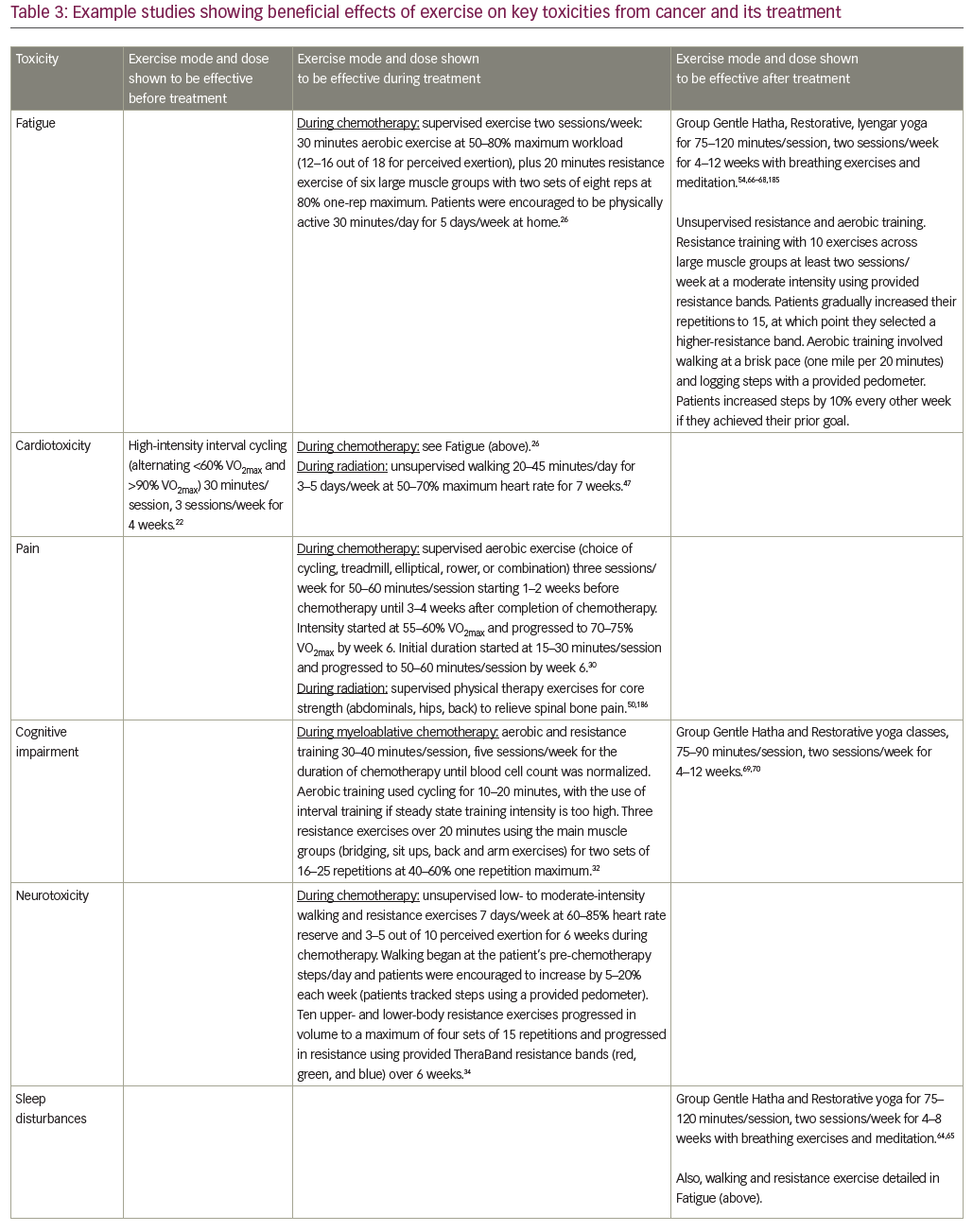
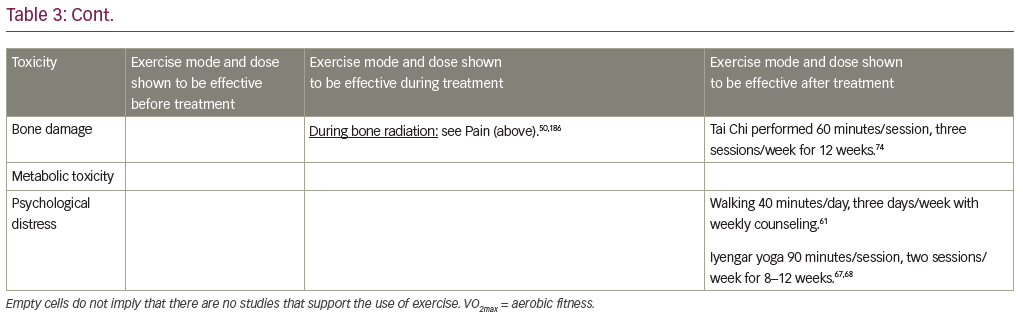
Clinical exercise interventions are often aimed at promoting recovery following surgery (rehabilitation) when patients are typically physically and mentally drained following their procedure. In contrast, “prehabilitation” increases the physiological function of a patient prior to surgery, enhancing tolerance to surgery and subsequent recovery.16 Prehabilitation is important because poor baseline functional reserve increases the risk of postoperative complications following major surgery.17 The feasibility and utility of exercise as a single mode of prehabilitation has been explored in a couple of RCTs and other clinical trials in patients undergoing surgery for cancer resection.18–22 These studies ranged in sample size from 30–112, and showed beneficial effects of 2–6 weeks of aerobic exercise or aerobic plus resistance exercise on quality of life and aerobic capacity measured by an aerobic capacity (VO2max) test or 6-minute walk test. For example, one RCT in 38 patients with liver metastases from colorectal cancer showed that supervised high-intensity interval cycling of 30 minutes/session, three sessions/week for 4 weeks, improved pre-surgical VO2max and quality of life compared to standard care.22 In another RCT of 112 patients scheduled to receive colorectal surgery, simple walking and breathing exercises improved walking capacity (6-minute walk test) both before and after surgery, compared to a cycling and strengthening intervention.20 These results may be due to the fact that the walking and breathing exercises yielded better adherence than cycling and strengthening (90% versus 16%), highlighting the importance of selecting an exercise intervention that a given sample will adhere to. Together, the prior research suggests that exercise prehabilitation is a feasible, safe, and effective way to improve preoperative fitness, provided appropriate exercises are selected (left column of Table 3). However, with only a few prior studies, further research with standardized outcomes is required to determine the optimal duration and mode of delivery before definitive recommendations can be made.
Exercise during cancer treatment
There are several RCTs demonstrating that exercise is safe, feasible, and effective in mitigating most of the NCORP high-priority toxicities (Table 1) when it is prescribed concurrently with chemotherapy, hormonal therapy, or radiation therapy (middle column of Table 3).
Exercise during chemotherapy
A recent meta-analysis,23 systematic review,24 and Cochrane review25 found that exercise significantly reduced fatigue both during or after completion of adjuvant chemotherapy. This exercise-induced improvement in fatigue was largest in studies using only aerobic exercise, although combined aerobic plus resistance training was effective too.23 For example, a recent three-arm RCT of 230 patients with breast cancer receiving adjuvant chemotherapy (the (the Physical Exercise During Adjuvant Chemotherapy Effectiveness Study [PACES])) compared (1) usual care, (2) an unsupervised low-intensity walking program of at least 30 minutes/day, 5 days/week, and (3) conditioned plus supervised moderate- to high-intensity aerobic plus resistance exercise program of 50 minutes/session, two sessions/week.26 The high-intensity exercise program reduced general fatigue more than usual care, and it reduced physical fatigue more than the low-intensity walking program. Several smaller RCTs suggest that fatigue during chemotherapy can be treated using yoga, with 60 patients with breast cancer receiving either standard care or yoga performed for 60 minutes/session, two sessions/week for 8 weeks,27 and Tai Chi, with 96 patients with lung cancer receiving either low-impact exercise (control) or Tai Chi performed for 60 minutes/session, every other day for 12 weeks.28
For cardiotoxicity, the higher-intensity aerobic and resistance exercise routine in the PACES trial significantly improved endurance and maximal short exercise capacity, assessed using a graded bicycle test, compared to usual care.26 In a separate study of 269 patients with mixed tumor types in both adjuvant and palliative chemotherapy settings, high-intensity resistance, aerobic, and relaxation exercise improved VO2max by 10.7% compared to usual care, which showed no change.29 Another study in 301 patients with breast cancer (the Combined Aerobic and Resistance Exercise [CARE] trial) showed that high-dose aerobic exercise (50–60 minutes/session, three sessions/week) increased VO2max more than low-dose aerobic exercise (25–30 minutes/session, 3 sessions/week).30
Both low- and high-intensity exercise interventions are effective in reducing pain in patients receiving chemotherapy, according to results from the PACES trial.26 Aerobic exercises may be particularly effective for pain control in patients receiving chemotherapy, as suggested by the 301 patients with breast cancer in the CARE trial, showing that high-dose aerobic exercise reduced pain more than combined aerobic plus resistance exercise.30 Cognitive impairment was improved by exercise in at least two studies. One was an RCT of 479 patients receiving chemotherapy comparing unsupervised low–moderate intensity walking and resistance exercise, when compared to usual care for 6 weeks.31 Another was an RCT of 48 patients receiving myeloablative chemotherapy followed by autologous stem cell transplant, wherein subjects were randomized to 40 minutes/day of ergometric and resistance exercises, had lower rates of cognitive impairment and exhibited better psychosocial function compared to patients in the physiotherapy control condition.32
For chemotherapy-induced peripheral neuropathy (CIPN), exercise significantly improved patient-reported numbness and tingling in an RCT comparing a 6-week unsupervised low–moderate intensity walking and resistance exercise routine to usual care in 355 patients receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy.33,34 In a separate trial of 60 patients with lymphoma receiving chemotherapy, a supervised moderate-intensity aerobic, resistance, and sensorimotor exercise intervention of 60 minutes/session, 2 sessions/week, significantly improved balance and reduced neuropathy symptoms compared to standard care.35
In regards to second-tier toxicities patients may experience while receiving chemotherapy (Table 1), aerobic and/or resistance exercise has been shown to improve sleep36 and self-esteem;37,38 however, several studies have demonstrated no improvement in depression and anxiety.27,38
Exercise during radiation therapy
Several RCTs have shown that many forms of exercise reduce fatigue during radiation treatment in patients with breast cancer39–41 and patients with prostate cancer.39,42–44 One study tested supervised moderate-intensity aerobic exercise for three sessions/week, at 50–60% VO2max using a cycle ergometer, treadmill, or elliptical; or supervised moderate-intensity resistance exercise for three sessions/week, with 10 exercises, at 60–70%, of their one-repetition maximum (independently tested) versus usual care, for 24 weeks, in 121 patients with prostate cancer.42 Another study tested yoga versus brief supportive therapy for 60 minutes/session, 3–7 sessions/week, for 6 weeks in 80 patients with breast cancer.45 Exercise RCTs have also shown improvements in cardiovascular fitness during radiation treatment in patients with breast cancer (e.g. 46 patients randomized to supervised moderate-intensity cycling or usual care control; 20 patients randomized to unsupervised moderate-intensity walking or placebo stretching control),46,47 whereas some studies have shown no effects (e.g. 126 patients randomized to unsupervised home-based walking or usual care).48 The null findings from Griffith et al.48 might have been because the exercise dose was insufficient (e.g., intensity was too low), as the other studies tracked exercise intensity objectively, using heart rate monitors. In one study examining cardiovascular fitness in patients with breast cancer receiving radiation therapy, 7 weeks of aerobic exercise increased VO2max, red blood cell count, and hemoglobin count, whereas patients randomized to the placebo stretching group became worse on all three measures.47 These results show that not only can exercise protect against treatment-induced toxicities, but exercise can actually improve cardiovascular fitness during cancer treatments.
The treatment of radiation-induced pain with exercise is also well-studied, with several RCTs showing benefits.40,48–50 One study in 60 patients showed that 2 weeks of resistance training, performed during radiation therapy, reduced patient-reported pain and opiate medication use both 3 months and 6 months after completion of radiation treatment, compared to passive physical therapy control.50 The use of exercise to treat cognitive impairment and neurotoxicity from radiation has not been well studied in humans, but several RCTs in animal models have shown that exercise protected against radiation-induced cognitive impairment when radiation was delivered to the brain.51 RCTs in humans have shown that exercise is effective for many second tier radiation-induced toxicities as well, including sleep problems (e.g. insomnia),41 bone damage (e.g. sparing radiation-induced bone loss in patients with bone metastases),52 metabolic toxicity (e.g. reducing appetite loss),41 and psychological distress (e.g. reducing negative effects and anxiety).45,53
Exercise during hormonal therapy
During treatment with aromatase inhibitors, exercise has proven helpful in reducing joint pain,54,55 bone pain,54,55 and bone damage,56,57 and exercise has been suggested to treat cognitive impairment based on successful treatments in animal models.58 In men receiving androgen deprivation therapy for prostate cancer, an RCT of 155 patients demonstrated that a 12-week resistance exercise routine improved fatigue, quality of life, and upper and lower body muscular fitness, when compared to usual care, although no improvement was seen in weight, body mass index, or waist circumference.59
Exercise after completion of cancer treatment (survivorship)
A growing body of evidence suggests that exercise is safe and effective in reducing toxicities experienced after completion of cancer treatments (right column of Table 3). As reviewed below, studies have tested aerobic exercise, combined aerobic and resistance exercise, and mindfulness-based exercise.
Aerobic exercise
One RCT in 86 breast cancer survivors showed that a 12-week unsupervised low–moderate intensity, home-based walking program, significantly reduced fatigue and exhibited a trend toward improving overall mood and body esteem.60 Another RCT in 116 lung cancer survivors who walked 3 days/week for 40 minutes/day with weekly counseling, improved their anxiety and depression levels.61
Combined walking and strength training
An RCT in 66 patients with stage IV lung or colorectal cancer post-treatment showed that an 8-week home-based resistance band exercise program, combined with incremental walking, improved the mobility, fatigue, and sleep quality compared to usual care.62 In another study, a 12-month exercise program (6 months of supervised aerobic and resistance exercise, followed by 6 months of home-based exercise maintenance) improved the physical function and health-related quality of life in 100 older long-term prostate cancer survivors compared to the physical activity education control condition.63
Mindfulness-based exercise
Several studies have investigated the effects of yoga on cancer-related toxicities such as sleep disturbances,64,65 fatigue,54,64,66–68 psychological distress,68 cognitive impairment,69,70 musculoskeletal symptoms,54 and menopausal symptoms.64 An RCT of yoga in 410 cancer survivors, compared standard care to standard care plus a 4-week program called Yoga for Cancer Survivors (YOCAS©®), which includes breathing exercises, postures, and meditation for 75 minutes/session, two sessions/week for 4 weeks.65 YOCAS significantly improved sleep quality,65 fatigue,54 global side-effect burden,54 memory difficulties,70 and musculoskeletal pain and discomfort,54 compared to standard care. A separate RCT comparing 12 weeks of Iyengar yoga for 90 minutes/session, two sessions/week, to health education among 31 breast cancer survivors showed that yoga reduced fatigue and depression.67 Another study in 37 breast cancer survivors, showed that an 8-week yoga program consisting of postures, breathing exercises, meditation, study of pertinent topics, and group discussion for 120 minutes/session, one session/week, significantly improved sleep disturbance, fatigue, and menopausal symptoms (e.g. hot flash, joint pain, menopause-related distress), compared to wait-list control.64 Several studies of one RCT in 21 breast cancer survivors demonstrated that a 12-week Tai Chi intervention performed for 60 minutes/session, three sessions/week, improved aerobic capacity, muscular strength, flexibility, body composition, self-esteem, quality of life,71–73 and bone health (increases in bone formation, decreases in bone resorption), compared to psychosocial support control.74
Biological mechanisms of toxicities and the effects of exercise
Although a complete review of mechanisms of exercise is out of the scope of this review, we highlight several biological mechanisms involved in toxicities from cancer and its treatment as well as the effects of exercise. These pathways include inflammation,8,9 endocrine hormones,9–11 the HPA axis,9,12 and mitochondria.13 Exercise can exert beneficial effects on all of these pathways simultaneously, partially explaining how exercise can have widespread beneficial effects on several cancer- and treatment-related toxicities.
It is well known that the immune system, including multiple inflammatory signaling pathways, is adversely affected by cancer,75 chemotherapy,76,77 radiation,78 and surgery.79 Although inflammation can be part of the healthy healing process, excessive chronic inflammation might lead to fatigue,80,81 pain,82 cognitive impairment,83 neuropathy,84,85 sleep disturbances,86 bone damage,87 metabolic toxicities (muscle wasting, cachexia),88,89 and psychological distress (anxiety, depression).88,90 Inflammation is mediated by an array of small proteins called cytokines, which act as inter-cellular signals that are secreted by various tissues in response to foreign invaders, ingested foods/drugs, stress, exercise, etc. Cytokines circulate in the blood and then bind to cell-surface receptors, whose activation triggers cascades of genetic and biochemical events related to the cell cycle, immune function, and inflammation.91 Inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 receptor agonist (IL-1RA), and interleukin (IL)-6 have been implicated in the development of cancer-related fatigue,80 cancer-related depression and anxiety (along with IL-8),90 and cancer cachexia.89,92–95 Chronic immune activation leads to dysfunction of the endocrine system, HPA axis, mitochondria,80 and dysregulation in the secretion of pro-inflammatory cytokines.80,88 The systemic effect of chronic immune activation adversely alters hormone release, cell respiration, and ultimately skeletal muscle synthesis and function, which may alter production of ghrelin and reactive oxygen species.
Ghrelin is a peptide hormone that is an endogenous ligand to the hypothalamic growth hormone (GH) secretagogue receptor (i.e. binding of ghrelin to this receptor induces the release of GH).96 GH release increases the amount of circulating insulin-like growth factor 1 (IGF-1)97 which, when bound to its receptor IGF-1R, signals the phosphatidylinositol-3 kinase (PI3K)/Akt pathway. Mammalian target of rapamycin (mTOR), the downstream target of the PI3K/protein kinase B (Akt) pathway, is phosphorylated and thus increases protein synthesis and inhibits protein degradation.97 Dysfunction of the endocrine system and HPA axis might interfere with this pathway, thus contributing to major cancer- and treatment-related metabolic toxicities.
Mitochondrial abnormalities that lead to oxidative stress, caused by increases in reactive oxygen species, have been linked to distress (e.g. depression) and muscle wasting.98–101 Depression involves dysregulation of monoamine neurotransmitters such as serotonin and norepinephrine, and hypersensitivity of the HPA axis.88 Specifically, research suggests that alterations in these pathways are related to an enhanced inflammatory response.88,92,102
Exercise is a promising treatment for many cancer-related toxicities because exercise affects multiple biological pathways. In particular, exercise has potent and well-studied anti-inflammatory effects.8 During exercise, IL-6 is released by contracting muscles in healthy individuals as well as cancer patients.103 This pro-inflammatory cytokine exerts anti-inflammatory effects by inhibiting the production of TNF-α and IL-1,104,105 and increasing production of anti-inflammatory IL-10. Progressive resistance training has also been shown to upregulate transcription factors that increase muscle protein synthesis through the phosphorylation of mTOR.106 Indeed, both resistance and endurance training are capable of reducing disease-induced muscle proteolysis through enhanced phosphorylation of Akt and the Forkhead transcription factor (FoxO1). Phosphorylation of FoxO1 prevents its relocation into the nucleus where it upregulates the transcription of atrophy genes.107 Thus, the beneficial effects of exercise on fatigue, cardiotoxicity, pain, cognitive impairment, neurotoxicity, etc., as discussed previously, might occur partly through exercise-induced changes in inflammation.
Health disparities in cancer and exercise research
Underserved cancer patients and survivors are less likely to be physically active at every stage of the cancer continuum.108,109 Underserved individuals in this context include racial and ethnic minorities (African Americans, Hispanics, and Asian Americans) and vulnerable populations (low socioeconomic status, low educational attainment, those residing in rural communities, sexual and gender minorities, geriatrics). Racial minority patients with breast cancer are less likely to participate in physical activity after a diagnosis109 and their participation in exercise declines during treatment.110 Cancer survivors are more like to be physically active if they are white (52% were active) or Asian-American (48%) versus Hispanic (39%) or African-American (32%).111 Studies have also found that physical activity participation rates are very low for cancer patients who live in rural communities.112,113
Both structural and personal barriers limit physical activity in underserved populations.114 Structural barriers include neighborhood or community safety concerns,115–117 lack of sidewalks and physical activity facilities,115,118,119 and lack of physically active role models.116,120 Socioeconomic stratifications affect health outcomes and quality of life of underserved cancer patients and they perpetuate disparities in who decides to exercise. Personal barriers to exercise include lack of time,115,121 lack of motivation,115,118,122 tiredness/fatigue,115,123 lack of knowledge,123,124 health conditions,115,118 physical appearance concerns,116,120,123 cost of facilities,115,118,123,125,126 and lack of social support.115,120,124 Additionally, most cancer patients report that they do not discuss exercise with their oncologist or primary care physician as part of their cancer treatments,127,128 despite the patient’s interest in learning more about exercise,129 and despite the fact that exercise adherence is increased if clinicians are involved in making recommendations for exercise.130 This leaves significant room for improvement to help patients initiate and adhere to an exercise program. Notwithstanding those barriers, underserved individuals can benefit from exercise to manage toxicities from cancer and its treatment.131
Clinicians can have a significant impact on initiating and adhering to an exercise program, especially for underserved patients. Physicians should recognize that underserved cancer patients are less likely to exercise, so they can be proactive in their discussions with patients. To reach underserved populations, physicians can network and partner with their communities and stakeholders (e.g. churches, gyms). Partnering with their communities will not only build a mutual respect and trust especially among the underserved communities but will also provide physicians opportunities to educate other members of the community about exercise and cancer care. Of course, education alone will likely not change a patient’s exercise behavior; support and resources from the community and clinical team are crucial to improvement. For example, patient navigators can provide assistance to patients who may need transportation or child care. In addition, the combined efforts from local government, federal government, community health centers, community members, and law enforcement should ensure safe neighborhoods, install sidewalks, and improve access to exercise facilities with reduced or free cost for individuals in need.
Technology to enhance exercise tracking and adherence
Mobile technology is a rapidly developing and promising way to help patients exercise more by tracking their exercise and providing feedback to enhance motivation and adherence. Nearly two-thirds of adults in the United States own a smartphone132 and technological advancements have enabled these devices to monitor health behaviors and provide convenient feedback.133 Wearable digital activity trackers have similar capabilities but may have more consumer appeal than a smartphone. Some of these devices are fairly accurate for measuring steps walked, with one study showing wrist-worn activity trackers underestimating steps by 1–23%, smartphone applications underestimating steps by 6–7%, and pedometers overestimating steps by up to a mere 1%.134 However, for obtaining heart rate data, the current generation of wrist-worn devices do not perform well, with one study showing 95% of estimated heart rate values varying by 30 beats per minute under or over the gold-standard electrocardiogram.135
Mobile devices are also being incorporated into behavioral interventions in an approach called mobile health, or mHealth. This new breed of interventions utilize mobile computing and communication technologies for a range of functions, such as clinical decision support systems, data collection tools for healthcare professionals, and supporting health behavior change for management of chronic diseases such as obesity and diabetes.136 The use of mobile health interventions is a rapidly expanding area of research and practice,133,137,138 and has only recently been applied to cancer patients and survivors.139,140 Next, we review the use of mobile behavioral interventions in the general population and in cancer patients specifically.
Mobile health behavioral interventions in the general population
In healthy adults, dozens of studies have suggested that the use of a pedometer, a relatively minimalist mobile health intervention, significantly increases physical activity, decreases body mass index, and decreases blood pressure.141 Short-term studies have shown that more rigorous mobile health behavioral intervention results in modest improvements in weight loss in the general population.142,143 Recently, an RCT entitled Innovative Approaches to Diet, Exercise, and Activity (IDEA) examined the effectiveness of pedometers for long-term health behavior modifications.144 At the beginning of the study, all participants were prescribed a low-calorie diet and physical activity program, with group counseling sessions. At 6 months, they were randomized to either the standard intervention group, which utilized self-monitoring of diet and physical activity using a website, or the enhanced intervention group, which utilized digital activity trackers and accompanying web interface to monitor diet and physical activity. While both groups had significant improvements in body composition, fitness, physical activity, and diet, these improvements were not statistically significantly different between the two groups. Furthermore, among adults with a relatively high body mass index of 25–40 kg/m2, integration of digital activity trackers to a standard behavioral intervention resulted in less weight loss over 24 months. However, the use of digital activity trackers was not initiated until 6 months after the onset of intervention, which may have hindered how the technology was used.
Mobile health behavioral interventions among cancer patients and survivors
A recent systematic review examined interactive web-based interventions that aimed to increase patient empowerment and physical activity for various chronic conditions in the general population.145 This review identified seven elements that can be translated into mobile health recommendations for cancer survivors: education, self-monitoring, feedback/tailored information, self-management training, personal exercise program, and communication with either healthcare providers or patients (e.g. chat, email). These essential components may serve as the foundation for designing future interactive mobile health interventions for cancer survivors, with the ultimate goal to improve their health status and quality of life. Importantly, two recent studies demonstrated feasibility and improved physical activity levels from two mobile health interventions: a 6-week, web-based, behavioral modification program for adult cancer survivors,139 and a technology-based, 6-month lifestyle intervention via either telemedicine or text messaging, for patients with endometrial cancer.140
Suggestions for clinicians
A growing body of evidence shows that exercise is safe, feasible, and effective to ameliorate several side effects of cancer and its treatments along the cancer treatment continuum. Our six principles and clinical suggestions are summarized in Table 2. The literature provides best practice suggestions for clinicians to prescribe exercise for treating or preventing certain toxicities, but does not yet provide definitive guidelines in terms of exercise type, frequency, intensity, and duration for all toxicities.
Prior research has provided an excellent starting point for the dose of exercise recommended for cancer patients, including special considerations and suggested modifications.6,7 The American College of Sports Medicine indicates cancer patients and survivors should slowly progress to 150 minutes/week of moderate-intensity aerobic exercise, or 75 minutes/week of vigorous intensity exercise, combined with 2–3 days of strength training across all major muscle groups, plus regular stretching.6 Higher-intensity exercise has been shown to be more effective for certain toxicities (e.g. cardiotoxicity,29 fatigue26) but only if that exercise is feasible.20 These results are consistent with the idea of an inverted-U association between exercise response and exercise dose (training intensity, session duration), suggested in reviews of exercise in relation to cancer toxicities,146 cancer mortality,147 heart disease,148 and healthy aging149 (i.e. a moderate dose exercise is best). Thus, exercise recommendations should be started slowly and, optimally, individualized with the help of an exercise professional who is familiar with the needs of cancer patients. Table 3 lists specific exercise regimens shown to be beneficial in an RCT.
Conclusions
The advent of improved cancer screening techniques and new anti-neoplastic therapies have led to improved overall survival for patients with cancer over the past 20 years. Additionally, patients living with cancer can be more active due, in part, to improvements in supportive care and clinical trials that have challenged the concept of over-treatment. These phenomena have opened the door to studying the utility of exercise in patients with cancer on treatment and cancer survivorship. Biological mechanisms of both cancer and its treatments, specifically chemotherapy, radiation, and hormonal therapy, that lead to inflammation, muscle breakdown, and functional limitations can be targeted using exercise. Furthermore, clinical trials suggest that exercise can treat many high-priority toxicities (Table 1)—namely fatigue, cardiotoxicity, pain, cognitive impairment, neurotoxicity, sleep disturbances, bone damage, metabolic toxicity, and psychological distress—sometimes before, during, and after cancer treatment (example exercise regimens in Table 3). The development of wearable activity trackers and mHealth interventions has enhanced our ability to measure patient activity, and how it may affect cancer and treatment-related toxicities. Many of these studies include low-cost, home-based interventions that are convenient and achievable in these populations. Despite the seemingly accessible nature of exercise, disparities still exist in employing the use of exercise in underserved communities. As the multitude of benefits of exercise in patients with cancer continue to come forth, it is important for healthcare providers and community leaders to address and remove barriers that patients may face so interventions like exercise can reach and help more and more patients each day.










