Human epidermal growth factor receptor-2 (HER2)-positive breast cancer accounts for 15% of all breast cancers.1 This breast cancer subtype was historically associated with poor outcomes; however, the development of HER2-directed therapies has dramatically improved outcomes for patients with both early and advanced HER2+ disease.2 Currently, there are several types of HER2-targeted agents approved for the treatment of breast cancer, including monoclonal antibodies (mABs), small molecule tyrosine kinase inhibitors and antibody–drug conjugates. In this narrative review, we summarize the diagnosis and prognosis of early-stage HER2+ breast cancer, and current treatment approaches.
Trastuzumab and pertuzumab
Trastuzumab is an immunoglobulin G1 kappa light chain recombinant humanized HER2-targeted mAB first approved for the treatment of advanced breast cancer in 1998. The proposed mechanisms of actions of this mAB include HER2 degradation, antibody-dependent cellular cytotoxicity and mitogen-activated protein kinase pathway interference.3 Several studies have assessed the role of this mAB in early breast cancer, and are summarized in Table 1.4–7 In NSABP B-31/NCCTG N9831, 4,046 patients with operable HER2+ breast cancer were randomized to receive an anthracycline and taxane-containing regimen (AC-T) with or without trastuzumab in the adjuvant setting.4 With a median follow-up of 8 years, the addition of trastuzumab led to a significant improvement in overall survival (OS), from 75.2% to 84%, and disease-free survival (DFS), from 62.2% to 73.5%.4 In this study, over 90% of patients had lymph node involvement and about half of the patients had hormone receptor-positive disease.4 The BCIRG-006 study included 3,222 women with early HER2+ breast cancer who were randomized to one of three adjuvant arms: arm A = AC-T; arm B = AC-T + trastuzumab (AC-TH); arm C = the anthracycline-free regimen docetaxel, carboplatin and trastuzumab (TCH).5 Patients enrolled in trastuzumab-containing arms received trastuzumab after chemotherapy to complete 52 weeks.5 At a median follow-up of 10.3 years, there was a DFS benefit for both trastuzumab-containing arms compared with the trastuzumab-free arm, and the hazard ratios (HRs) for AC-TH and TCH were relatively close (0.64 and 0.75, respectively).6 There was also an OS benefit for both trastuzumab-containing regimens.6 More events of heart failure were observed in the AC-TH arm (2%, n=21) versus TCH (0.4%, n=4), and seven out of the eight cases of acute leukaemia were reported in patients treated with anthracycline-containing regimens.5,6 In this study, about 70% of patients had lymph node involvement and about half had hormone receptor-positive disease.5 In the HERA study, 5,099 women were randomized to observation, or 1 or 2 years of adjuvant trastuzumab.7 At a follow-up of 11 years, the addition of a year of trastuzumab improved DFS and OS when compared with observation, whereas 2 years of trastuzumab did not seem to add significant benefits compared with 1 year of treatment.7 In this study, 68% of patients received an anthracycline-containing regimen and 26% received anthracycline- and taxane-containing regimens; about 57% of patients had lymph node involvement and 50% had hormone receptor-positive disease.7 Based on these studies, trastuzumab, in combination with adjuvant or neoadjuvant chemotherapy followed by trastuzumab to complete a year of therapy, has been the standard of care since 2006.
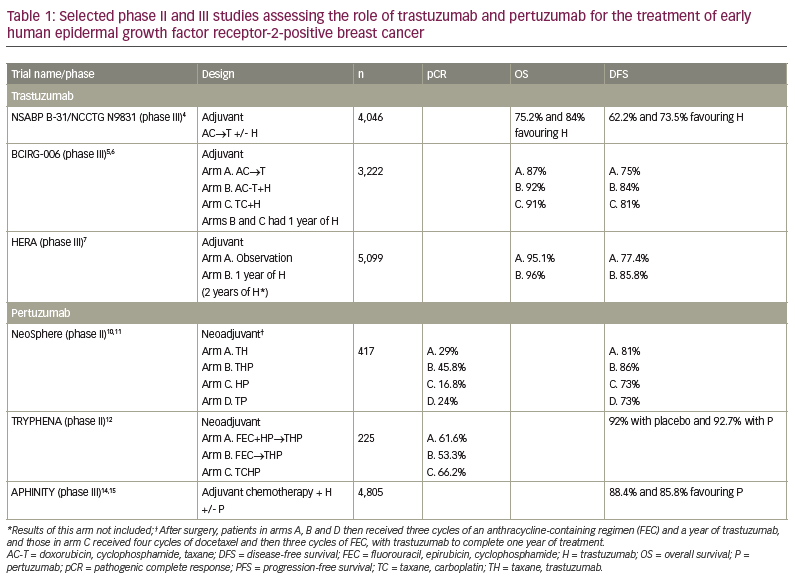
Pertuzumab is another recombinant humanized mAB that targets a different domain of the HER2 receptor from trastuzumab and prevents HER2–HER3 dimerization, reducing downstream pathway activation.8 This mAB was initially approved in combination with trastuzumab and chemotherapy for the treatment of metastatic HER2+ breast cancer in 2012, based on the findings of the CLEOPATRA trial.8,9 Several studies have assessed the role of pertuzumab in the treatment of early HER2+ breast cancer, and are summarized in Table 1.
NeoSphere was a phase II study in which 417 patients received four cycles of neoadjuvant therapy with: arm A = docetaxel and trastuzumab (TH); arm B = docetaxel, trastuzumab and pertuzumab (THP); arm C = trastuzumab and pertuzumab (HP); or arm D = docetaxel and pertuzumab (TP).10 After surgery, patients in arms A, B and D received three cycles of an anthracycline-containing regimen (FEC) and completed a year of trastuzumab, and those in arm C received four cycles of docetaxel and then three cycles of FEC followed by trastuzumab.10 The primary endpoint of this study was pathological complete response (pCR).10 Overall, 45.8% of patients treated with THP achieved a pCR, compared with 29% of those treated with TH, 24% with TP and 16.8% with HP.10 At a median follow-up of 5 years, the progression-free survival (PFS) was 81% for arm A, 86% for arm B, 73% for arm C and 73% for arm D.11 Patients who achieved a pCR, irrespective of the treatment arm, had a longer PFS than those with residual disease after neoadjuvant therapy (HR 0.54, 95% confidence interval [CI] 0.29–1.00).11 TRYPHENA was another phase II study in which 225 patients were randomized to receive: arm A = FEC+HP → THP; arm B = FEC → THP; or arm C= the anthracycline-free regimen TCHP.12 After surgery, all patients received trastuzumab to complete a year.12 The study assessed the cardiac safety of the combinations.12 Declines in left ventricular ejection fraction were noted in 5.6%, 5.3% and 3.9% of the patients, respectively.12 pCR was reported in 61.6% of the patients in arm A, 53.3% in arm B and 66.2% in arm C.12 Achievement of pCR has been shown to correlate strongly with long-term outcomes in patients with HER2+ disease.13 Based on the findings of NeoSphere and TRYPHENA, pertuzumab was approved in the neoadjuvant setting in 2013, and dual HER2-targeted agents in combination with chemotherapy became the standard of care.
APHINITY was a randomized, phase III trial in which 4,805 patients with HER2+ breast cancer were randomized to receive adjuvant chemotherapy and trastuzumab with or without pertuzumab.14 In this study, 78% of patients were treated with an anthracycline-containing regimen (FAC/FEC-TH or AC-TH), and 22% with an anthracycline-free regimen (TCH).14 Patients continued the same HER2-directed therapy (H or HP) to complete a year.14 Overall, 64% of patients had hormone receptor-positive disease, and in terms on lymph node status, 3% of patients had tumours <1 cm and node-negative disease, 34% had tumours >1 cm and node-negative disease, 38% had 1–3 lymph nodes involved, and 25% had 4 or more lymph nodes involved.14 The third planned interim analysis of APHINITY was recently presented:14 at a median follow-up of 8.4 years, the iDFS (primary endpoint) was 88.4% versus 85.8% favouring pertuzumab (HR 0.77, 95% CI 0.66–0.91), with a lower number of distant and locoregional recurrences in the pertuzumab arm.15 Most of the benefit was observed in the node-positive cohort, with an iDFS of 86.1% and 81.2% favouring pertuzumab (HR 0.72, 95% CI 0.60–0.87); there was no difference in the node-negative cohort (93.3% and 92.3%, HR 1.01, 95% CI 0.72–1.42).15 There was also no difference in iDFS by hormone receptor status.15 At 8.4 years, the OS in the intention-to-treat population was 92% with placebo versus 92.7% with pertuzumab (HR 0.83, 95% CI 0.68–1.02; p=0.78).15 When assessing the cohort with lymph node-positive disease, the OS was 89.2% versus 91.1%, respectively (HR 0.80, 95% CI 0.63–1.00).15 Conversely, in the node-negative cohort, the OS was 96.4% with placebo versus 95.5% with pertuzumab (HR 0.99, 95% CI 0.64–1.55).15 There was a less than 1% rate of cardiac events in both arms, with 19 events (0.8%) in the pertuzumab arm and 10 events (0.4%) in the placebo arm. Based on these findings, pertuzumab in combination with trastuzumab and chemotherapy is the standard of care for patients receiving neoadjuvant therapy, and pertuzumab in combination with trastuzumab is the standard therapy for patients who had nodal involvement at the time of the diagnosis and who achieve a pCR with neoadjuvant therapy. Even though the abovementioned studies dramatically changed the outcomes of patients with early HER2+ breast cancer, several questions regarding the optimal treatment of this population remain unanswered.
Should all patients with early HER2+ breast cancer be treated with dual HER2-targeted monoclonal antibodies and combination chemotherapy?
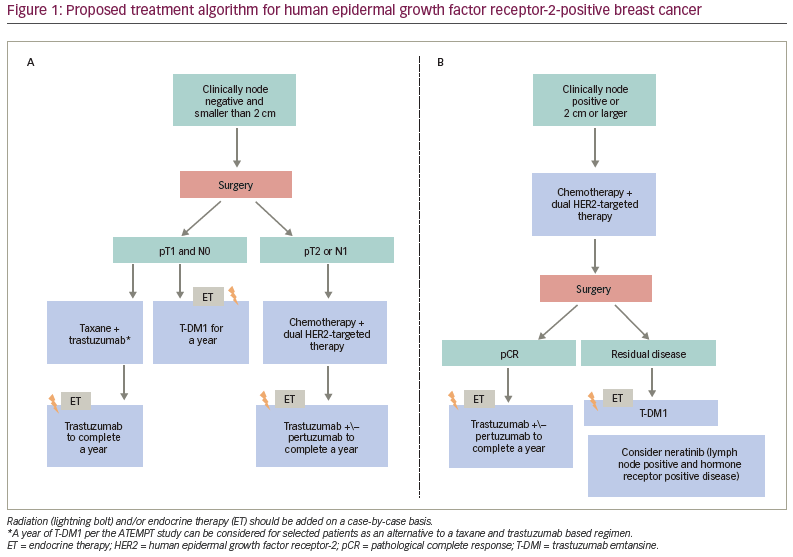
Several studies have shown the feasibility of de-escalation of therapy for patients with anatomically low-risk disease. The Adjuvant paclitaxel and trastuzumab (APT) trial was a single-arm study in which 406 patients with tumours measuring up to 3 cm and without nodal involvement received 12 weeks of adjuvant paclitaxel and trastuzumab (TH) followed by trastuzumab monotherapy to complete a year.16 With this de-escalated adjuvant regimen, the 10-year DFS was 91.3% and the OS was 94.3%.17 There was no difference in outcomes based on hormone receptor status, tumour grade or tumour-infiltrating lymphocytes.17 Given that patients with low-risk disease had excellent long-term outcomes with this therapy, adjuvant paclitaxel and trastuzumab is the current standard of care for patients with small, lymph node-negative tumours. Figure 1A shows the current treatment algorithm for stage 1 HER2+ breast cancer.
Similarly, the phase II ATEMPT trial randomized 497 patients with stage 1, HER2+ breast cancer to the APT regimen or to a year of the antibody–drug conjugate trastuzumab emtansine (T-DM1).18 The study was not designed to compare iDFS between the treatment arms but rather to establish the 3-year iDFS for 1 year of T-DM1 and to compare toxicities between the two arms.18 The 5-year iDFS was 97% for T-DM1, with a recurrence-free interval of 98.6% and OS of 97.8%, and clinically relevant toxicities were not statistically different between the arms; however, patients receiving T-DM1 reported better quality of life, and less neuropathy and alopecia than those receiving the APT regimen.18,19 ATEMPT proved that T-DM1 is an alternative to the APT regimen in selected patients, and has comparable efficacy and toxicity profiles.18 The ongoing ATEMPT 2.0 study (NCT04893109)20 is comparing toxicities between the APT regimen to six cycles of T-DM1 followed by trastuzumab to complete a year; while ADEPT (NCT04569747)21 is assessing the role of endocrine therapy in combination with trastuzumab and pertuzumab for patients with stage 1, hormone receptor- and HER2-positive breast cancer. In summary, patients with stage 1 disease can receive adjuvant therapy with TH or with T-DM1; both regimens are associated with excellent long-term outcomes.
A frequent clinical question is whether some patients with clinically node-negative disease and tumours smaller than 2 cm could benefit from neoadjuvant chemotherapy. Importantly, up to 24.7% of patients with T1c and clinically node-negative disease are found to have lymph node metastases at the time of surgery; these findings suggest that presurgical imaging with ultrasound should be considered for patients with clinically stage 1 disease.22 These patients could potentially benefit from neoadjuvant therapy; therefore, in selected patients with larger T1c tumours (1.5–2 cm) and high-risk features, neoadjuvant chemotherapy with HER2-targeted therapies could be considered.
Should all patients with T2 or node-positive tumours be treated with neoadjuvant therapy?
Neoadjuvant therapy provides several benefits for patients with HER2+ breast cancer. In addition to potential downstaging to facilitate improved surgical outcomes, it allows for assessment of response to therapy.13 The assessment of response to therapy also allows us to tailor adjuvant therapy; patients who achieve a pCR continue treatment with trastuzumab with pertuzumab, whereas those with residual disease have been shown to derive a significant benefit by an escalation with adjuvant T-DM1, based on the findings of the KATHERINE study.23 KATHERINE was a phase III study in which 1,486 patients with residual disease after neoadjuvant therapy were randomized to receive adjuvant trastuzumab versus T-DM1.23 After 3 years of follow-up, the iDFS was 88.3% with T-DM1 and 77% with trastuzumab (HR 0.50, 85% CI 0.39–0.64; p=0.001); the risk of recurrence was decreased by 50% with T-DM1.23 Based on these findings, neoadjuvant chemotherapy in combination with dual HER2-directed therapy is now the standard of care for patients with stage 2–3 breast cancers. Figure 1B shows the current treatment algorithm for stage 2–3 HER2+ breast cancer.
What is the optimal neoadjuvant chemotherapy backbone?
As previously discussed and summarized in Table 1, several treatment combinations have been studied for the treatment of HER2+ breast cancer in the (neo)/adjuvant settings. Based on the findings of NeoSphere, TRYPHENA and APHINITY, there is a clear benefit of combining a multi-chemotherapy regimen with HER2-targeted mABs.10–12,15 BCIRG 006 showed that the anthracycline-free regimen TCH was associated with good DFS and OS outcomes and with lower rates of cardiomyopathy when compared with AC-TH.6 TRYPHENA showed that the anthracycline free-regimen TCHP was associated with high pCR rates and a lower incidence of cardiac events when compared with anthracycline-containing regimens.12 More recently, the phase III, TRAIN-2 study assessed whether adding anthracyclines improved pCR rates in patients with HER2+ early breast cancer.24 This study included 438 patients with stage 2–3 breast cancer, randomized to receive an anthracycline-free or an anthracycline-containing regimen. No significant difference in pCR rates was observed with the two regimens.24 Additionally, at a median follow-up of 3 years, the event-free survival rate was 93% in those treated with and 94% in those treated without anthracyclines; the results were irrespective of hormone receptor or nodal status.25 The OS rate was 97.7% in the anthracycline-free arm and 98.2% in the anthracycline-containing arm.25 A decrease in left ventricular ejection fraction was noted in 7.7% of the patients treated with anthracyclines versus 3.2% of those who did not receive this treatment.25 Additionally, two patients treated in the anthracycline arm developed acute leukaemia.25 Based on these findings, it is reasonable to consider anthracycline-free chemotherapy regimens in combination with HER2-targeted mABs, a choice that is currently endorsed by the latest National Comprehensive Cancer Network guidelines.
Should patients with tumours larger than 2 cm and clinically node-negative disease receive neoadjuvant dual HER2-targeted monoclonal antibodies?
The update from APHINITY confirmed the role of pertuzumab in improving outcomes only for patients with node-positive disease at the time of diagnosis, with an absolute improvement of 1.9% in OS and 4.9% in iDFS when compared with pertuzumab-free regimens,15 now with a median follow-up of 8.4 years. Theoretically, this treatment could be used exclusively for patients with lymph node involvement. However, assessing the lymph node status before initiating neoadjuvant therapy is challenging. A recent study showed that up to 24.7% of patients with HER2-positive, T1c and clinically node-negative disease who go for surgery upfront are found to have lymph node metastases.22 The main risks of giving pertuzumab include a higher rate of diarrhoea and financial toxicity; this drug has not been associated with increased rates of cardiac toxicities. Given that pertuzumab is clearly associated with improved outcomes and it is hard to assess the nodal status definitively prior to administering preoperative therapy, the standard of care for patients with stage 2–3 disease includes chemotherapy with trastuzumab and pertuzumab. Ongoing studies are assessing whether de-escalation of chemotherapy while continuing dual HER2-targeted mABs is associated with good long-term outcomes and fewer toxicities; these studies include Decrescendo (NCT04675827)26 and CompassHER pCR (NCT04266249),27 both using a taxane with dual-HER2 blockage in the neoadjuvant setting.
Should we select (neo)/adjuvant HER2-targeted therapy based on hormone receptor status?
About 50% of tumours expressing HER2 positivity also express oestrogen/progesterone receptors. It has been well described that patients with hormone receptor- and HER2-positive breast cancer have a lower rate of pCR.10,25 Data from APHINITY, however, showed no difference in outcomes of iDFS or OS by hormone receptor status.15 To date, we do not tailor the neoadjuvant therapy based on hormone receptor status. Endocrine therapy is added to the adjuvant portion of treatment, once standard chemotherapy is completed, and continued for 5–10 years.
The optimal endocrine therapy for premenopausal patients with tumours that express hormone receptors and HER2 remains unclear. A recent analysis from the Short-HER trial showed that out of the 853 patients enrolled in the study, 40% were premenopausal, of whom 55% received tamoxifen, 22% aromatase inhibitors, 15% a combination, and half received ovarian function suppression (OFS).28 At a median follow-up of 7 years, the DFS was better for premenopausal patients who received aromatase inhibitors compared with those who received tamoxifen or combination therapy (87.3% versus 81.7%; HR 1.46, 95% CI 1.05–2.03; p=0.017).28 Studies such as SOFT and TEXT, which compared tamoxifen alone, tamoxifen with OFS and aromatase inhibitor with OFS in premenopausal women with hormone receptor-positive disease, included small numbers of patients with HER2+ disease (n=695, 10.3%).29 These studies have demonstrated improved outcomes with OFS and endocrine therapy for premenopausal women with high-risk disease. While data are limited for HER2+ disease, it is common practice to provide these therapies for patients with high-risk disease irrespective of the HER2 status.
What is the optimal treatment for those who achieve a pathological complete response and for those with residual disease after neoadjuvant therapy?
For patients who achieve a pCR, the optimal HER2-directed therapy is represented by anti-HER2 mABs. In APHINITY, patients received adjuvant therapy, and therefore, the lymph node status was known at the time of randomization.15 In current clinical practice, most patients with stage 2–3 disease receive neoadjuvant therapy, and therefore it is challenging to know with certainty the lymph node status at diagnosis. Given this challenge, many patients receive adjuvant pertuzumab and trastuzumab if they achieve a pCR after neoadjuvant therapy.8 For patients with residual disease after neoadjuvant therapy, the treatment of choice is T-DM1, based on the abovementioned KATHERINE study.23 For patients with hormone receptor-positive disease, endocrine therapy should be added to the adjuvant therapy with trastuzumab, trastuzumab/pertuzumab or T-DM1.
Another question is the role of extended HER2-directed therapy with neratinib (a pan-HER reversible small molecule inhibitor), the only HER2-targeted tyrosine kinase inhibitor currently approved for the treatment of early breast cancer. ExteNET was a phase III trial in which 1,334 patients were treated with a year of neratinib after completing adjuvant trastuzumab.30 At the 5-year follow-up, iDFS was 90.2% with neratinib versus 87.7% with placebo (HR 0.73, 95% CI 0.57–0.92; p=0.0083).29 The benefit was greater in patients with lymph node involvement, and the benefit was only seen in patients with hormone receptor-positive disease.30 One of the limitations of this study is that patients had not received prior pertuzumab or T-DM1; the benefit of neratinib in patients treated with these agents remains unclear. Diarrhoea is a common adverse event of neratinib; in ExteNET, 55% of patients developed grade 1–2 diarrhoea and 40% had grade 3.30 In the CONTROL trial, several anti-diarrhoea prophylaxis and dose-escalation regimens were used with the goal of improving neratinib tolerance in the ExteNET patient population; both anti-diarrhoea prophylaxis and dose-escalation regimens decreased grade, rate and severity of diarrhoea.31 Neratinib can be considered for patients with residual lymph node involvement and hormone receptor-positive disease; all patients should receive anti-diarrhoea prophylaxis and/or a dose-escalation regimen to decrease the severity of diarrhoea. The ongoing CompassHER2-RD trial (NCT04457596)32 assesses the role of T-DM1 with or without the HER2-specific tyrosine kinase inhibitor, tucatinib, in patients with residual disease after six cycles of chemotherapy, and findings may help elucidate the role of tyrosine kinase inhibitors in this setting.
Can we select patients based on biological markers and not only nodal status and tumour size?
There is growing interest in identifying prognostic and predictive biomarkers beyond tumour size, nodal status and breast cancer subtype, with the goal of tailoring patients’ treatment to intensify or de-escalate therapy based on tumour biology. Some examples include the use of circulating tumour DNA (ctDNA) and gene expression profiling. Regarding ctDNA, an analysis of the NeoALTTO study showed that detection of ctDNA before neoadjuvant therapy was associated with lower rates of pCR.33 Although monitoring ctDNA is not currently used to guide clinical decisions, ongoing studies aim to determine which interventions could prevent relapses of patients with detectable ctDNA.
Gene expression assays play a key role in selecting which patients with early hormone receptor-positive/HER2-negative disease derive a benefit from chemotherapy, and their use has reshaped the treatment paradigm of this breast cancer subtype.34,35 The 27-gene HER2DX test incorporates four gene signatures to provide a pCR score and a survival score.36 This assay was recently validated using samples from the Short-HER study;28 this analysis showed that HER2DX could be used as a predictive marker of recurrence, and the disease risk evaluated with HER2DX could be used in the future to tailor neoadjuvant therapy for patients with early HER2+ breast cancer.36 Additionally, this assay was also used in the APT and ATEMPT trials.17,19 Both studies confirmed that HER2DX was significantly associated with iDFS and recurrence-free interval.17,19 A cutoff of 32 was optimal for distinguishing between low- and high-risk patients in the APT trial.17 Prospective studies are needed to validate this assay, and further work is needed to determine how to use it to guide therapeutic decisions.
There are several ongoing trials assessing treatment de-escalation, such as MARGOT (NCT04425018),37 Decrescendo26 and CompassHER pCR27 neoadjuvant studies. Another example is ADEPT,21 which is assessing a chemotherapy-free adjuvant regimen comprising endocrine therapy in combination with trastuzumab and pertuzumab for patients with stage 1, hormone receptor- and HER2-positive breast cancer. Other studies are assessing the treatment intensification for patients at higher risk for recurrence, such as CompassHER2 RD,32 which is investigating whether the addition of the irreversible HER2-selective tyrosine kinase inhibitor, tucatinib, to adjuvant T-DM1 for patients with residual disease after neoadjuvant therapy improves long-term outcomes, while DESTINY-Breast05 (NCT04622319)38 is comparing T-DM1 versus trastuzumab deruxtecan in a similar patient population. Finally, Astefania (NCT04873362)39 is assessing whether the addition of the immune checkpoint inhibitor, atezolizumab, to T-DM1 can improve outcomes for patients with high-risk disease. The same immune checkpoint inhibitor has already been tested in the neoadjuvant setting within the IMpassion050 trial.40 IMpassion050 was a phase III study in which 454 patients with early HER2+ breast cancer were randomized to receive an anthracycline- and taxane-containing neoadjuvant regimen in combination with trastuzumab, perutuzumab and paclitaxel (dose-dense AC-THP) with or without atezolizumab.40 This study showed no difference in the pCR rates between the treatment arms. There were five fatal adverse events in the atezolizumab arm and the trial was ultimately discontinued due to an unfavourable benefit–risk ratio. The Astefania trial is using a single-agent T-DM1 backbone, which may make the treatment more effective and safer. The ongoing clinical trials with additional biomarker analyses will likely allow us to continue personalizing the treatment for patients with early HER2+ breast cancer.
Conclusions
The landscape of the treatment of HER2+ breast cancer is evolving rapidly. Currently, we can tailor treatment based on tumour size, lymph node status and response to neoadjuvant therapy. However, future research is needed, including other predictive and prognostic biomarkers with the goal of continuing to improve patient outcomes and decrease exposure to unnecessary treatments for patients with a good prognosis.