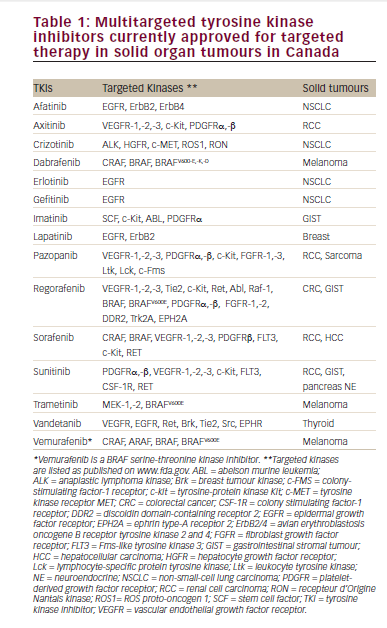
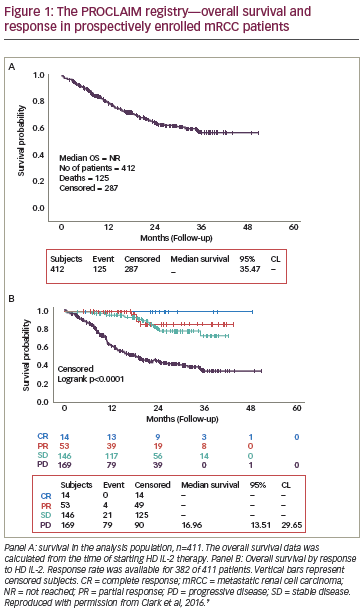
The last decade has seen significant advances in the treatment of renal cell carcinoma (RCC), both in immunotherapy and targeted agents. Recently, the results of two landmark clinical trials, Checkmate 025 and METEOR, have demonstrated the efficacy and safety of nivolumab and cabozantinib, respectively, in the second-line treatment of RCC. In this expert interview, Ulka Vaishampayan of Wayne State University, Detroit, Michigan, discusses the findings of these studies, their implications for clinical practice, and other recent therapeutic developments in RCC.
Q: Could you tell us a little about the findings of the Checkmate 025 study?
The Checkmate trial compared nivolumab with everolimus, and demonstrated overall survival (OS) benefit in advanced RCC previously treated with anti–vascular endothelial growth factor (VEGF) therapy.
Q: Could you tell us a little about the findings of the METEOR study?
The METEOR study was the registration trial for cabozantinib in RCC and led to the US Food and Drug Administration (FDA) approval in advanced RCC previously treated with a VEGF receptor (VEGFR) inhibitor. This was a pivotal phase III study that was conducted in advanced RCC patients, pretreated with VEGFR inhibitors.1 Patients were required to have a clear-cell component, performance status of 0–2 and adequate liver, renal, and bone marrow function. In this open label, randomized phase III trial, adult patients with advanced or metastatic clear-cell RCC, measurable disease, and previous treatment with one or more VEGFR tyrosine-kinase inhibitors (TKIs), were randomized to receive oral 60 mg cabozantinib daily, or 10 mg everolimus once a day. Stratification factors consisted of Memorial Sloan Kettering Cancer Center risk group, and number of prior therapies with VEGFR TKIs. The primary endpoint was progression-free survival (PFS) as assessed by an independent radiology review committee in the first 375 randomly assigned patients with an anticipated hazard ratio (HR) of 0.667, and the study was expanded to a total of 658 patients to evaluate the OS endpoint. The secondary endpoint was objective response rates.1 Cabozantinib treatment resulted in improved PFS (HR 0.51 [95% confidence interval [CI] 0.41–0.62]; p<0.0001) and objective response (17%) favoring cabozantinib as compared with 3% with everolimus; p<0.0001) per independent radiology review. Median PFS was 7.4 months with cabozantinib as compared with everolimus with 3.8 months. The median duration of follow-up for OS and safety at last report was 18.7 months. Median OS was 21.4 months (95% CI 18.7–not estimable) with cabozantinib and 16.5 months (14.7–18.8) with everolimus (HR 0.66 [95% CI 0.53–0.83]; p=0.00026).1
Q: How will the results of these studies inform future treatment strategies for renal cell carcinoma?
Results of these well-conducted randomized trials present two options with improved OS, nivolumab or cabozantinib, in the second-line therapy of RCC.
Q: What do you consider the other most significant recent therapeutic developments in renal cancer?
CABOSUN was conducted by the Alliance for Clinical Trials in Oncology as part of Exelixis’ collaboration with the National Cancer Institute’s Cancer Therapy Evaluation Program. The results of this study reveal that cabozantinib improved the response rate, PFS, and OS as compared with that achieved with sunitinib in the intermediate- and poor-risk patient population with clear-cell RCC. The 57 patients with bone metastases were a subset that consistently demonstrated a HR of 0.51 favoring cabozantinib.2 An independent review is ongoing to assess the response and progression results within CABOSUN and if this confirms the investigator-assessed results, then FDA submission of cabozantinib can be anticipated for the first-line therapy of metastatic RCC.
The CABOSUN results have the potential to change the entire therapeutic paradigm in advanced RCC, where cabozantinib will be used first-line and followed by consideration of other VEGFR TKIs or immune therapy. However, first-line immune therapy combination trials are also expected to report results within the next 12 months. Two large randomized trials include the combination of ipilimumab+ nivolumab as compared with sunitinib as the control arm, and the combination of bevacizumab and atezolizumab also compared with sunitinib in untreated advanced RCC. Whether in the first-line or second-line setting, the dilemma in systemic therapy decisions will remain between the use of immune therapy or TKI-based therapy. Predictive biomarkers to help guide therapy will be of critical importance.
Q: How is the treatment paradigm for renal cell carcinoma likely to evolve in the next few years?
The therapies and the sequence thereof will most likely change in RCC. The first-line therapy will likely be an immune therapy combination such as ipilimumab and nivolumab or bevacizumab and atezolizumab, as both these combinations have completed phase III trials in comparison with sunitinib. There will be a domino effect in the second-line setting, depending on which first-line therapy is chosen. Multiple novel agents continue to be tested in clinical trials and hopefully biomarker-driven therapeutic choice will be validated in the future.