In 1971, Folkman formulated the hypothesis that tumour growth and metastasis are angiogenesis-dependent events. 1 Vascular endothelial growth factor (VEGF) plays a key role in angiogenesis. VEGF levels have been demonstrated to be elevated in choroidal melanoma.2,3 However, the use of anti-angiogenic agents such as bevacizumab – a recombinant humanised anti-VEGF monoclonal antibody – has not been extensively investigated for the treatment of choroidal melanoma.
In 1971, Folkman formulated the hypothesis that tumour growth and metastasis are angiogenesis-dependent events. 1 Vascular endothelial growth factor (VEGF) plays a key role in angiogenesis. VEGF levels have been demonstrated to be elevated in choroidal melanoma.2,3 However, the use of anti-angiogenic agents such as bevacizumab – a recombinant humanised anti-VEGF monoclonal antibody – has not been extensively investigated for the treatment of choroidal melanoma.
The impact of intravitreal bevacizumab as a primary therapy for choroidal melanoma and its role in the management of complications (e.g., radiation retinopathy) from established therapies (e.g., brachytherapy) will be discussed here. Results from animal models, in which bevacizumab has been used either systemically or intravitreally, will be reviewed. Current clinical trials looking at the administration of intravitreal or systemic anti-VEGF agents for choroidal melanoma will be summarised.
Treatment of Primary Tumour
Intravitreal bevacizumab, along with other anti-VEGF agents, has gained widespread use in ophthalmology over the last few years for the treatment of neovascular diseases, such as age-related macular degeneration, central retinal vein occlusion, proliferative diabetic retinopathy and macular oedema. It has been shown to reduce neovascular activity and vascular permeability in ocular tissues.4,5
Nevertheless, despite the overexpression of VEGF in uveal melanoma,2 at present no anti-angiogenic drug has been shown to clinically suppress tumour growth.6 Currently, standard therapies include enucleation, local resection or radiotherapy, either by proton beam irradiation, stereotactic or brachytherapy (iodine or ruthenium). The efficacy of brachytherapy in primary tumour control approaches 95 %.7
We reported a series of three patients who inadvertently received multiple intravitreal injections of bevacizumab (1.25 mg in 0.05 ml) for presumed choroidal neovascularisation, but who were later diagnosed with choroidal melanoma. No evidence of the drug halting tumour progression was observed. Instead, the use of bevacizumab led to the masking of the underlying tumour, thereby delaying accurate diagnosis. In two of the cases, enucleation was performed and bevacizumab-induced fibrotic retinal changes were evident on histopathology.8 One of these two cases is illustrated in Figures 1–4. It is that of a 55-year-old woman who presented for further investigation of a peripapillary choroidal lesion presumed to be an idiopathic choroidal neovascular membrane, and who was treated with five intravitreal injections of bevacizumab at monthly intervals.
Choroidal melanoma has intrinsic vessels and commonly has associated subretinal fluid. Bevacizumab may transiently diminish tumour vasculature and permeability, reducing the subretinal fluid. However, subretinal fluid resolution may be misleading in this scenario and the tumour may to continue to grow.8
In the absence of studies documenting the efficacy of bevacizumab for the treatment of choroidal melanoma, caution is advised in the use of this drug as a sole treatment modality.
Management of Complications
Since the Collaborative ocular melanoma study (COMS), therapy for medium-sized choroidal melanomas has shifted from enucleation to plaque brachytherapy, as the survival rate has been found to be similar.7
Radiation retinopathy, optic neuropathy, iris neovascularisation and neovascular glaucoma are potential complications of plaque brachytherapy. The risk of these complications is related to the presence of systemic disease (e.g., type 1 diabetes), tumour location and thickness, radiation dose and the use of radiation sensitisers (e.g., chemotherapy).9–12
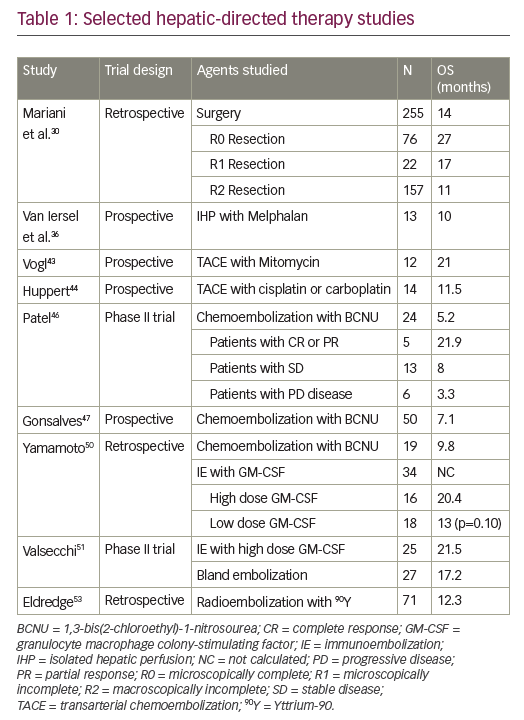
Radiation retinopathy has been reported to occur in up to 63 % of patients after plaque brachytherapy and generally occurs following the first year of treatment.13 The result is both retinal exudation (oedema, hard exudates and retinal haemorrhages) and ischaemia (cotton wool spots and capillary dropout). Histologically, there is an obliterative endoarteritis, with loss of vascular endothelial cells and pericytes, capillary closure and thickened vessel walls, leading to neovascularisation.9 If ischaemia is present, VEGF likely has a role in the pathogenesis of the retinopathy.13 It has been hypothesised that bevacizumab may decrease ocular ischaemia resulting from plaque brachytherapy, as well as vascular permeability in non-vital tissue.14 A few case series have been published regarding the use of bevacizumab (1.25 mg in 0.05 ml) in patients with radiation retinopathy following plaque brachytherapy (see Table 1). No systemic side effects were observed. Improvement of the macular oedema has been demonstrated in those studies, but most patients required multiple injections for a sustained effect. Moreover, the improvement in visual acuity after injection appears to be modest and is seen only in few patients, limiting the role of bevacizumab.14–17
Finger reported the use of bevacizumab in a patient who developed radiation optic neuropathy 18 months after having been treated for a juxtapapillary choroidal melanoma with a notched palladium-103 plaque. At six weeks, decreased haemorrhage and optic disc oedema were documented. Visual acuity improved from 20/32 to 20/20 within one week.18 A recently published series also reported reductions in disc oedema and haemorrhages after the use of intravitreal bevacizumab in 14 patients who developed radiation optic neuropathy related to plaque radiotherapy for choroidal melanoma. Vision was stable or improved in nine of the 14 patients.19 A limitation of the study was the absence of a control group to evaluate the natural history of radiation optic neuropathy.
The use of bevacizumab for neovascular glaucoma developing as a complication of plaque brachytherapy has also been documented. Within one week of the injection, regression of iris and angle neovascularisation was observed, and intraocular pressure control was achieved with the use of topical glaucoma medications. Visual acuity remained unchanged.20
Animal Models
Although current therapy for medium-sized uveal melanomas, such as plaque brachytherapy, provides an excellent control rate of the primary tumour, mortality from metastatic disease has remained unchanged for decades.21,22
In choroidal melanoma, metastasis occurs via the haematogenous route, most commonly to the liver. Melanoma cells have been shown to secrete VEGF and angiogenesis has been demonstrated in uveal melanoma and its metastasis.2,3 It has been speculated that inhibition of VEGF may diminish the metastatic potential by avoiding angiogenesis, providing a possible role for bevacizumab.23 However, a large variation of tumour VEGF expression has been reported in uveal melanoma and expression of VEGF may correlate with poor prognosis.24,25
Yang et al. demonstrated a decreased number of hepatic micrometastases in a mouse ocular melanoma model after intraperitoneal injection of bevacizumab (50 or 250 μg/100 μl). The effect was greater with early treatment (Day 1 after inoculation of melanoma cells), possibly indicating that micrometastases had not yet become established.23 The authors’ experiments, in contrast to what we observed in our clinical series,8 also demonstrated a dose-dependent decrease in the size of the primary tumour after the use of bevacizumab. However, the drug had no effect on inducing apoptosis of the malignant cells and tumour reduction was thought to be secondary to diminished intratumoral endothelial cell proliferation, causing a shrinking effect on tumour volume.23
In another uveal melanoma mouse model, intraocular injection of bevacizumab (2 μg/4 μl) led to acceleration of intraocular tumour growth. Moreover, the drug increased messenger RNA VEGF melanoma expression in vitro, particularly under hypoxic conditions. The paradoxical upregulation of VEGF upon anti-VEGF treatment has been described in other tumours and may represent a tumour adaptive response.6
Current Clinical Trials
There are several clinical trials using bevacizumab or ranibizumab (a monoclonal antibody fragment derived from the same mouse antibody as bevacizumab) that are currently being conducted. The summary information of relevant trials is given below.
- Intravitreal bevacizumab for large uveal melanoma (ClinicalTrials.gov identifier NCT00596362) – A clinical trial is underway examining the potential of intravitreal bevacizumab to cause a clinically significant reduction in tumour size in eyes scheduled to undergo enucleation. Results have not been published yet.
- Intravitreal ranibizumab for the prevention of radiation maculopathy following plaque radiotherapy for choroidal melanoma (ClinicalTrials.gov identifier NCT00540930) – A trial is being conducted wherein intravitreal ranibizumab (0.5 mg) is injected at the time of radioactive plaque insertion. The primary outcome measure is grade of macular oedema on optical coherence tomography and visual acuity at four months. Results have not been published yet.
- Ranibizumab in combination with proton beam irradiation for choroidal melanoma (ClinicalTrials.gov identifier NCT00765921) – The purpose of this study is to investigate the safety and tolerability of ranibizumab in combination with proton beam irradiation for the treatment of choroidal melanoma by determining the incidence and severity of ocular and systemic adverse events. The study is currently recruiting patients.
- Temozolomide and bevacizumab in treating patients with metastatic uveal melanoma (ClinicalTrials.gov identifier NCT01217398) – This Phase II study of first-line treatment for metastatic uveal melanoma is recruiting patients, with the primary outcome measure of objective response rate and stable disease rate at six months.
Conclusions
Despite the widespread use of intravitreal bevacizumab, along with other anti-VEGF agents, the role of the drug in choroidal melanoma appears to be limited. Currently, no study has demonstrated efficacy of bevacizumab in tumour control in humans. Caution is strongly advised regarding the use of bevacizumab as a single primary modality of treatment in uveal melanoma. Additional role(s) of bevacizumab in the management of uveal melanoma, radiation complications following treatment of uveal melanoma, and metastatic uveal melanoma are under investigation. ■