Head and neck cancer (HNC) will affect more than an estimated 55,000 individuals in the US in 2014, the vast majority of these being squamous cell carcinoma (SCC), with 12,000 estimated deaths.1 Worldwide, the figures for incidence and mortality are approximately 560,000 and 300,000, respectively.2 The current standard of care for local-regionally advanced SCC of the pharynx and larynx is chemoradiotherapy with a platinumbased agent, most commonly high-dose cisplatin (100 mg/m2 q 3 weeks), and increasingly weekly cisplatin (30–40 mg/m2). In the last decade or so, targeted therapies against EGFR have also been studied in this patient population. EGFR is a receptor tyrosine kinase (TK), a member of the ErbB family of receptors, and is activated by binding of an epidermal growth factor and other specific ligands. Once activated, EGFR transitions from an inactive monomer to an active homo- or heterodimer, and thereby stimulates the downstream intracellular protein-TK activity, which can ultimately result in hallmarks of carcinogenesis, namely cell proliferation, blocked apoptosis, invasion and metastasis, and tumor-induced neovascularization.3 It is now well-known that mutations that lead to constitutive activation and ultimately overexpression of EGFR are associated with several distinct malignancies.4,5 Heretofore, six EGFR inhibitors have been approved by the US Food and Drug Administration (FDA) for use in non-small cell lung carcinoma; pancreatic carcinoma; HER2-overexpressing breast cancer; metastatic colorectal carcinoma (EGFR-overexpressing); and SCC of the head and neck. The inhibitors belong to one of two categories— monoclonal antibodies (e.g., panitumumab and cetuximab) are intravenous agents whose mechanism of action is through extracellular binding of the EGFR with subsequent inhibition of downstream signaling pathways, whereas the TK inhibitors (e.g., erlotinib, gefitinib, and lapatinib) are oral agents whose mechanism of action is through intracellular binding and subsequent inhibition of downstream signaling pathways. The first EGFR inhibitor approved in 2004 by the FDA for use in SCCHN is a monoclonal antibody agent cetuximab (Erbitux® ImClone, Bristol-Myers Squibb). Besides its proven in vitro and in vivo radiosensitization, its toxicity profile is also relatively and comparably favorable: it effects varying degrees of dermatologic toxicity, modest gastrointestinal toxicity with electrolyte abnormalities, and is also nonemetogenic.6– 8
EGFR Inhibition in Combination with Radiotherapy as Definitive Treatment of SCCHN
Concurrent chemoradiotherapy (CRT) improves survival and organ preservation for patients with local-regionally advanced SCCHN.9–13 The National Comprehensive Cancer Network (NCCN) Guidelines maintain that the standard CRT approach for fit patients with locally advanced disease is upfront concurrent high-dose cisplatin and radiotherapy (Category 1 recommendation based upon high-level evidence and with uniform NCCN consensus). Due to toxicity with high-dose cisplatin, other concurrent systemic agents that have a more favorable toxicity profile, and are thereby easier for patients to tolerate, have been studied.14,15 Results of several phase II and III trials testing cetuximab as part of an intensified treatment approach for patients with SCCHN, whether as an addition to induction chemotherapy (IC) or to the concurrent CRT, have been published in recent years. The best known of these trials is a phase III study conducted by Bonner and colleagues that demonstrated that cetuximab in combination with radiotherapy also improves survival compared with radiation alone for patients with SCCHN.16,17 In this trial, 424 patients with stage III–IV SCC of the oropharynx, hypopharynx, or larynx were randomized to radiotherapy with or without cetuximab. The original publication reported that after a median follow-up of 4.5 years, a 10 % absolute overall survival (OS) benefit was observed in the concurrent cetuximab arm (45 % versus 55 %). Local-regional control was also significantly better with cetuximab (47 % versus 34 %). Of note, the patients who benefitted the most from receiving cetuximab were also treated with a concomitant boost radiation course, an accelerated fractionation approach that has been shown in a phase III randomized trial (RTOG 9003) to effect significantly improved local-regional control compared with the standard once-daily fractionation for locally advanced HNC.18 Moreover, the subgroup analysis favored radiotherapy alone in patients with a Karnofsky Performance Status (KPS) 60–80, and in patients 65 years or older. The highly anticipated RTOG (1016) trial comparing CRT with high-dose cisplatin versus bioradiotherapy (BRT) with cetuximab in patients with human papilloma virus (HPV)-related SCC of the oropharynx just completed accrual. The primary objective of this trial is to determine whether substitution of cisplatin with cetuximab will result in comparable 5-year OS.19
EGFR Inhibition as Part of Induction Regimen in Patients with SCCHN
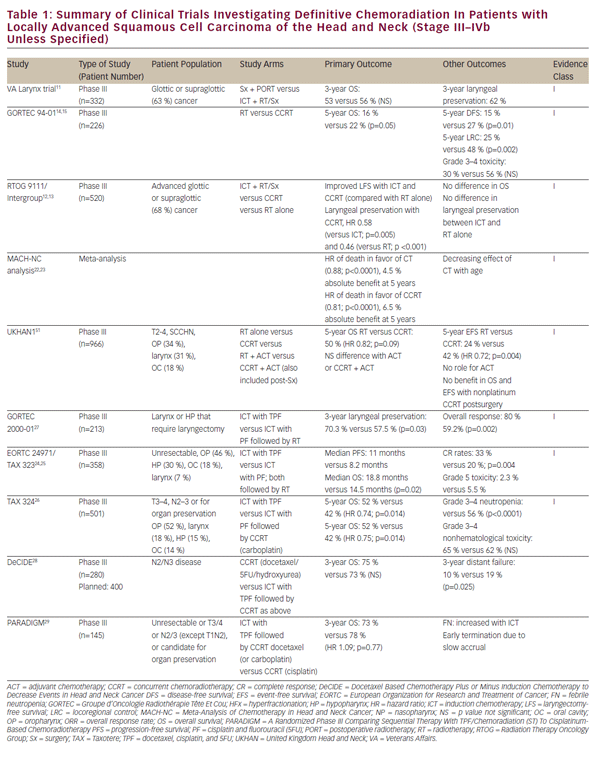
Randomized phase III studies comparing sequential chemotherapy/ radiotherapy to concurrent CRT alone have failed to demonstrate a survival benefit with the incorporation of IC, but this approach remains popular in Europe and elsewhere.20,21 However, the risk for distant metastases tends to correlate with site and the extent of nodal involvement at presentation. As concurrent CRT regimens continue to improve local-regional control and survival, there may be a counterpart increase in the risk for developing distant metastases, especially in patients with involvement of multiple ipsilateral cervical nodes or contralateral nodal involvement, which remains the rationale for IC.22 Probably the best-known clinical trial incorporating IC is the VA Larynx trial that effected a paradigm shift in the treatment of locally advanced laryngeal cancer, as well as the TAX 324 trial, which demonstrated that the addition of docetaxel to cisplatin/5-fluorouracil (TPF) followed by CRT improved OS (62 % versus 48 % at 3 years) in patients with local-regionally advanced SCCHN.23,24 Given the toxicity of TPF, combination of other systemic agents, often incorporating an EGFR inhibitor, have been tested in more-recent phase II trials and reported institutional series.
Kies and colleagues at the MD Anderson Cancer Center reported the results of an institutional phase II trial of combination cetuximab plus IC comprised six weekly cycles of paclitaxel–carboplatin in patients with advanced SCCHN, precisely those with nodal disease N2b-N3, followed by risk-based local therapy, which consisted of either radiation alone, concurrent CRT, or surgery.25 This regimen was deemed feasible, effective, and well tolerated, with a promising progression-free survival (PFS) and OS (87 % and 91 %, respectively) at a median-follow-up time of 33 months. Not surprisingly, patients with HPV-positive tumors had a superior PFS and OS compared with patients with HPV-negative tumors. Argiris and colleagues reported results of a phase II trial combining induction therapy with docetaxel, cisplatin, and cetuximab followed by definitive therapy with radiotherapy (70 Gy to gross disease), weekly cisplatin and cetuximab, and maintenance cetuximab for 6 months.26 Out of 39 enrolled patients, 36 had stage IVA/B disease and 23 an oropharyngeal primary. With a median follow-up of 36 months, 3-year PFS and OS were 70 % and 74 %, respectively. Eight patients developed a local-regional recurrence, three developed distant recurrence, and one both. The toxicities were reported to be more manageable than the published toxicities in the landmark studies utilizing induction TPF.
EGFR Inhibition as Part of Concurrent Regimen in Patients with SCCHN
Recently published results from the RTOG 0522 trial suggest that adding cetuximab to CRT with high-dose cisplatin did not improve outcomes in more than 800 patients with advanced SCCHN, and according to the report, this combination should not be prescribed routinely.27 Cetuximab plus CRT, versus CRT alone, resulted in more-frequent interruptions in radiation therapy (26.9 % versus 15.1 %, respectively); similar cisplatin delivery (mean, 185.7 mg/m2 versus 191.1 mg/m2, respectively); and more grade 3 to 4 radiation mucositis (43.2 % versus 33.3 %, respectively), but not more late toxicity. No differences were found between Arms A and B in 3-year PFS (61.2 % versus 58.9 %, respectively; p=0.76), 3-year OS (72.9 % versus 75.8 %, respectively; p=0.32), local-regional failure (19.9 % versus 25.9 %, respectively; p=0.97), or distant metastasis (13.0 % versus 9.7 %, respectively; p=0.08). Patients with p16-positive oropharyngeal carcinoma, compared with patients with p16-negative tumors, had better 3-year probability of PFS (72.8 % versus 49.2 %, respectively; p<0.001) and OS (85.6 % versus 60.1 %, respectively; p<0.001), but tumor EGFR expression did not distinguish outcome. The toxicity analysis of the addition of cetuximab to concurrent CRT comprising the paclitaxel-carboplatin doublet was evaluated in a prospective phase II institutional study by Birnbaum and colleagues, who demonstrated that addition of cetuximab increases dermatologic toxicity (grade 3–4 acneiform rash in 6 % versus 3 % of patients), but does not increase mucosal toxicity compared with previous studies of an identical regimen without cetuximab.28 The efficacy of this combined approach was evaluated in a phase II study as reported by Suntharalingam and colleagues, wherein after a median follow-up of 31 months in 43 patients with stage III/IVA/IVB SCCHN, the complete response (CR) at the completion of therapy was 84 %, with an estimated 3-year local-regional control rate of 72 %. The actuarial OS and diseasefree survival (DFS) at 3 years were 59 % and 58 %, respectively.29 In a similarly designed bicentric phase II trial (REACH Trial), the interim analysis of addition of cetuximab to CRT regimen (carboplatin and 5-fluorouracil with accelerated fractionation radiotherapy in the form of concomitant boost) in 43 previously untreated patients with local-regionally advanced SCCHN, revealed that the treatment was tolerated moderately well, with an objective response rate of 28.6 % CR at first follow-up and 92.9 % thereafter.30 Specifically in local-regionally advanced larynx and hypopharynx carcinoma, the recent randomized phase II study (TREMPLIN) evaluated larynx preservation (LP) at 3 months with IC with docetaxel, cisplatin and fluorouracil (TPF) followed by either CRT (with cisplatin every 3 weeks) or BRT (with weekly cetuximab) in 116 patients.31 Difference in LP at 3 months was not significant between the two arms (95 % and 93 %, respectively), nor was larynx function preservation (87 % versus 82 %, respectively) and OS (92 % versus 89 %, respectively) at 18 months. At 18 months, however, fewer local (with or without regional) failures were recorded in the CRT arm than in the BRT arm (8 % versus 14 %), and this was observed after a median follow-up of 36 months, as well (13 % versus 21 %, respectively); of note, successful salvage surgery was achieved in the BRT arm only. Regional and distant metastases were similar in the two arms. In terms of toxicity, there was no difference between arms in grade 3 to 4 mucositis, but the BRT arm had double the grade 3 in-field skin toxicity. Also, significantly more patients in the CRT arm required protocol modification due to limiting acute toxicity after intensive TPF induction (43 % versus 71 % patients received the full protocol, respectively).
A recently reported interim analysis from a large European multicenter 2×2 factorial study suggested that induction TPF chemotherapy did not compromise compliance with the subsequent concurrent CRT. At the American Society of Clinical Oncology (ASCO) meeting 2014 in Chicago, the investigators presented the interim results on 415 patients with locally advanced SCCHN who were randomized to one of four treatment arms: Arm A1: cisplatin-5FU doublet x two cycles concomitant with conventional radiotherapy versus Arm A2: cetuximab plus radiotherapy; Arm B1: three cycles of TPF followed by the same CRT as Arm A1; Arm B2: three cycles of TPF followed by cetuximab and radiotherapy.32 After a median follow-up of 41.3 months, median PFS was 29.7 months in induction versus 18.5 months in the concurrent arm with a 3-year PFS of 46.8 % versus 36.7 % (hazard ratio [HR] 0.73; p=0.015), respectively. Median OS was 53.7 months in the induction arm versus 30.3 months in the concomitant arms, with a 3-year OS of 57.6 % versus 45.7 % (HR 0.72; p=0.025), respectively. They concluded that induction TPF followed by CRT with a cisplatin doublet, or combination of cetuximab and radiotherapy, significantly improved PFS and OS in this patient population, and without compromising compliance with the concurrent portion of treatment.
As mentioned above, one of the ways to inhibit EGFR downstream intracellular signaling is with a TK inhibitor (TKI), whose site of action is on the inner cell membrane. Erlotinib is one such TKI approved by the FDA as therapy for advanced pancreatic and non-small cell lung cancer. Its use in preclinical models suggests potential synergism with cisplatin, and it has been shown to effect a significant disease control when combined with cisplatin in patients with recurrent/metastatic disease.33,34 In a randomized phase II trial, Martins and colleagues evaluated 204 patients with stage III or IVA/B SCCHN to receive the standard definitive treatment of high-dose q 3 weeks cisplatin and radiotherapy for 7 weeks (to 70 Gy) with (Arm B) or without (Arm A) erlotinib.35 The primary endpoint was CR rate, as evaluated by central review (negative visualization of the primary site, or negative biopsy when indicated, and no residual node on computed tomography [CT] greater than 15 mm or with necrotic center or negative neck dissection). Patients who received cetuximab (Arm B) had a CRR of 52 %, whereas patients who received only CRT had a CRR of 40 % (p=0.08). Toxicity was not increased with the addition of erlotinib to CRT. After a median follow-up of 26 months and 54 progression events (25 [46 %] in Arm A and 29 [54 %] in Arm B), there was no statistical difference in the PFS between the two arms. Erlotinib administration had a high rate of compliance and did not compromise the administration of cisplatin or completion of radiotherapy.
Cohen and colleagues assessed efficacy and toxicity of gefitinib, another EGFR inhibitor, in the setting of two cycles of induction carboplatin-paclitaxel doublet, then gefitinib with concurrent CRT comprising fluorouracil, hydroxyurea, and twice-daily radiotherapy, followed by continued gefitinib for 2 years total. CRR, as assessed by Response Evaluation Criteria in Solid Tumors (RECIST) 1 month after completion of concurrent CRT in the 66 patients they studied was 90 %; all patients had stage III or IVA/B SCC of the tongue base or hypopharynx. After a median follow-up of 3.5 years, the 4-year OS, PFS, and disease-specific survival (DSS) rates were 74 %, 72 %, and 89 %. In this series, high EGFR gene copy number was associated with significantly worse OS.36
EGFR Inhibition as Part of Adjuvant Treatment in Patients with SCCHN
A substantial proportion of patients with SCCHN undergo primary surgery. Certain high-risk histopathologic features, namely presence of microscopically involved resection margin, multiple involved nodes, or extracapsular nodal extension of tumor, dictate the addition of postoperative CRT, a standard of care supported by two major phase III clinical trials, as well as a comparative analysis—RTOG-9501 and EORTC-22931.37–39 Harari and colleagues recently reported the results of the RTOG 0234, a randomized phase II trial examining concurrent CRT (with either weekly cisplatin 30 mg/m2 or weekly docetaxel 15 mg/m2) and cetuximab in the postoperative treatment of patients with SCCHN with any one or more of the aforementioned high-risk pathologic features.40 The treatment was deemed feasible and tolerable with predictable toxicity. After a median follow-up of 4.4 years of 238 enrolled patients, the 2-year OS was 69 % for the cisplatin arm and 79 % for the docetaxel arm; the 2-year DFS was 57 % and 66 %, respectively. The rate of grade 3 to 4 myelosuppression in the docetaxel arm was half that of the cisplatin arm (14 % versus 28 %), and rate of mucositis was essentially equivalent, circa 55 %. The DFS in this study was compared with the historical control in the CRT arm of the RTOG-9501, which had a HR of 0.76 for the cisplatin arm versus control (p=0.05) and 0.69 for the docetaxel arm versus control (p=0.01), reflecting absolute improvement in 2-year DFS of 2.5 % with cisplatin and 11.1 % with docetaxel.
The evaluation of incorporating cetuximab into the postoperative or adjuvant treatment of patients with high-risk histopathologic features continues in a currently open phase II/III trial (RTOG 1216) that evaluates 60 Gy radiotherapy plus concurrent cisplatin (control) versus 60 Gy plus docetaxel versus 60 Gy plus the combination of docetaxel and cetuximab. The objective of the phase II component is to select the better experimental arm to improve DFS over the control arm, and the subsequent phase III component is to determine whether the selected experimental arm will improve OS over the control arm.41
Adjuvant radiotherapy alone is the current postoperative standard of care in patients with ‘intermediate’ risk adverse features on histopathologic analysis of the surgical specimen, namely presence of any one or more of the following: perineural invasion, lymphovascular invasion, single or multiple involved lymph nodes without extracapsular extension, close surgical margins (defined as cancer extending to within 5 mm of resection margin), T3 or T4a primary tumor, or a T2 oral cavity cancer with >5 mm depth of invasion. With adjuvant radiotherapy alone, the rate of local-regional failure rate varies between 15–35%.42–44 Machtay and RTOG colleagues are currently conducting a phase III trial (RTOG 0920) to test whether the addition of cetuximab to radiation therapy will improve OS in postoperative patients with intermediate risk for recurrence following surgery.45
EGFR Inhibition in Recurrent or Distantly Metastatic SCCHN
The efficacy of cetuximab plus platinum-based chemotherapy as first-line treatment in patients with recurrent or metastatic SCCHN was investigated by Vermoken and colleagues in a phase III randomized trial.46 This relatively large trial enrolled 442 patients who underwent randomization. The addition of cetuximab was associated with a 2.7-month increase in the median survival (10.1 versus 7.4 months) and a significant 20 % reduction in the relative risk for death compared with platinum-fluorouracil chemotherapy alone. Secondary efficacy endpoints were also significantly improved in the cetuximab group, with a 2.3-month prolongation of PFS (a 46 % reduction in the risk for disease progression), an 83 % increase in the response rate, and a 41 % reduction in the risk for treatment failure. A similarly designed phase III trial conducted by Eastern Cooperative Oncology Group (ECOG) randomly assigned 117 patients with recurrent/ metastatic SCCHN to receive cisplatin every 4 weeks, with weekly cetuximab (Arm A) or placebo (Arm B).47 Median PFS was 2.7 months for Arm B and 4.2 months for Arm A. The HR for progression of Arm A to Arm B was 0.78 (95 % confidence interval [CI] 0.54 to 1.12). Median OS was 8.0 months for Arm B and 9.2 months for Arm A (p=0.21). Enhancement of response was greater for patients with EGFR staining present in less than 80 % of cells. Their conclusion was that the addition of cetuximab to cisplatin significantly improved response rate, and that there is a survival advantage for the development of a rash. PFS and OS, however, were not significantly improved by the addition of cetuximab in this patient cohort.
Argiris and colleagues reported the results of another phase III randomized, placebo-controlled ECOG trial of docetaxel with or without gefitinib in recurrent or metastatic SCCHN.48 Two hundred and seventy patients were enrolled before the study was closed early at interim analysis. Median OS was 6 months in patients who were treated with docetaxel alone versus 7.3 months in patients who received the combination treatment. An unplanned analysis showed improved survival in patients younger than 65 years who received gefitinib (median survival 7.6 versus 5.2 months; p=0.04). Except for grade 3–4 diarrhea, which was more common with docetaxel/gefitinib, the grade 3–4 toxicities were comparable between the two arms.
Future Directions
As oncologists treating patients with local-regionally advanced SCCHN, we now have Level One evidence supporting definitive radiotherapy with concurrent systemic therapy in the form of cisplatin, carboplatin doublet, or cetuximab. As this review has shown, both cisplatin and EGFR inhibitors like cetuximab in combination with radiotherapy are effective in enhancing tumor response, but it is unclear which patients would benefit from either agent in combination with radiotherapy. With the advent and FDA approval of a growing number of newer-generation EGFR inhibitors, it will become increasingly important to discern if and when these should be optimally incorporated into the treatment regimen. An exciting new European trial (Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcome [ARTFORCE]) is currently enrolling patients with advanced SCCHN as per its factorial 2 by 2 design: cisplatin versus cetuximab and standard radiotherapy versus redistributed radiotherapy to the metabolically most 18-fluoro-deoxyglucose positron emission tomography (FDG-PET) avid part of the tumor.49 In the ARTFORCE trial, the correlation of biological markers and median uptake on the zirconium-labeled cetuximab (89Zr-Cetuximab) with treatment specific outcomes will be analyzed. The hope is that the results of this trial, as well as much anticipated results from RTOG 0920, RTOG 1016, RTOG 1216, and other ongoing phase III trials, will serve as an unequivocal guide to a more customized and risk-based treatment for the individual patient with SCCHN.