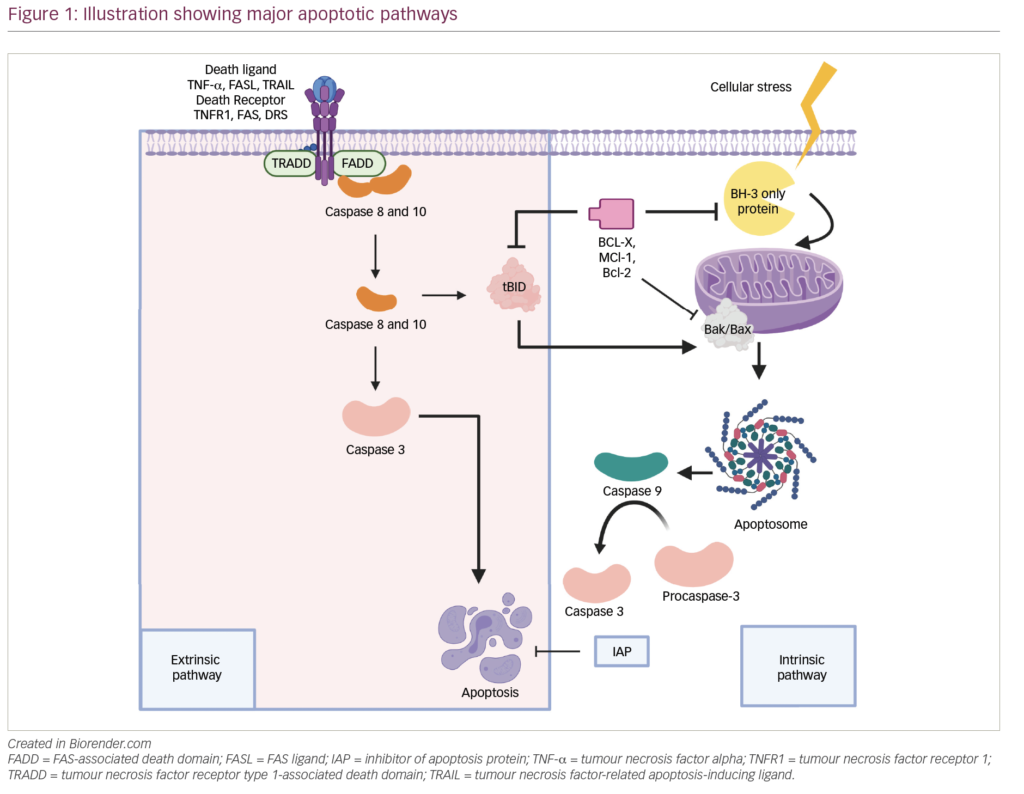
Multiple myeloma (MM) is manifested by abnormal clonal plasma cell proliferation and accounts for approximately 1 % of all cancers and nearly 10 % of hematologic malignancies in the US.1,2 It is considered to be incurable, probably as a consequence of the ongoing clonal evolution over the course of disease. Although the disease remains incurable, with the introduction of autologous hematopoietic stem cell transplant (AHSCT) and novel agents, such as thalidomide, bortezomib, lenalidomide, and carfilzomib, median overall survival (OS) has increased from nearly 3 years a decade ago to over 8 years currently.3,4 Advances in the understanding of disease biology and better stratification of risk groups according to the genetic abnormalities have led to the development of a risk-based individualized therapeutic approach.5 AHSCT is an effective therapy for MM and remains a standard of care in eligible patients.6 However, the majority of patients will have disease progression or relapse at some point after AHSCT or after the initial frontline therapy. Unfortunately, patients with high-risk disease based on genetic abnormalities as well as other laboratory features are likely to relapse even faster. Maintaining response after AHSCT is an important goal and the depth of the response correlates with improved long-term outcomes, especially progression-free survival (PFS).7–10 Trying to develop strategies in order to prevent or delay disease progression or relapse has resulted in the concepts of consolidation and maintenance therapies. Consolidation is a short-term therapy administered to enhance the rate and depth of a previously obtained response. By contrast, the goal of maintenance therapy is the extension of response duration, and ultimately of PFS and OS with the use of lower doses of drugs given with minimal toxicity over a long period of time.3 An optimal maintenance treatment should have the following characteristics: (1) should maintain and maybe deepen the response achieved after front-line therapy or AHSCT, (2) should prolong OS, (3) should be well tolerated and simple to administer (preferably as a single agent regimen and orally), with manageable drug-related toxicities, and no long-term deleterious effects, (4) should not decrease quality of life, and (5) should be cost-effective. This review summarizes the evolution and the current status of maintenance therapy in MM.
Initial Studies of Maintenance in Multiple Myeloma
Over the past 40 years, several maintenance strategies for MM have been investigated. Initial attempts in the 1960s to early 1980s were with single agent corticosteroids, continuous conventional chemotherapies, or interferon (IFN), many demonstrating the ability to prolong relapse-free survival but without prolongation of OS. Alexanian and colleagues evaluated various melphalan-based regimens in 508 patients with MM, randomly allocating 96 responders to one of three maintenance regimens, namely intermittent courses of carmustine with prednisone, continued courses of melphalan with prednisone (MP), or no chemotherapy. There were no differences in the relapse rate, the remission duration, or survival among these maintenance groups. The frequencies of pneumonia and herpes zoster were higher in patients receiving continuous chemotherapy.11
In the MRC trial, after 1 year of allocated treatment, 297 survivors were randomized to either stopping all treatment until relapse, or to continue treatment with azathioprine and vincristine, interrupted every 3 months for a course of the first-allocated treatment. The overall results demonstrated a trend toward benefit with maintenance, though the differences were not statistically significant. Most of the difference was found among a few patients with unfavorable prognostic features who survived 1 year and were eligible for randomization.12
Cohen et al. reported in 1986 on a randomized trial evaluating the impact of three approaches to consolidation and maintenance therapy after maximal response to initial therapy. All patients were treated initially with bis-chloroethylnitrosourea (BCNU), cyclophosphamide, and prednisone (BCP) until a designated level of response was achieved. Responders were randomly assigned to MP, prednisone, adriamycin, azathioprine, and vincristine (PAIV), or no therapy until relapse, followed by treatment with BCP at relapse. Initial response rates were similar to previous trials. A small number of incremental responses were observed with both MP and PAIV. Survival was the same for all three maintenance approaches. They concluded that additional or consolidation/maintenance therapy of the type administered in the study appears to offer little advantage once an initial response has been achieved.13 Two years later, in 1988, Belch et al. reported their randomized trial, which was designed to assess the role of maintenance MP in responding MM patients. One hundred and eightyfive eligible patients who responded to initial MP with stabilization for at least 4 months were randomized to either stop treatment and resume therapy at relapse or to continue MP until relapse. Time to first relapse was significantly shorter in the no maintenance arm; however, 57 % of the no maintenance patients achieved a second response when MP was restarted. Median survival from the start of MP in the maintenance group (46 months) was not significantly different compared with the no maintenance group (51 months). Multivariable analysis demonstrated shorter total remission duration and shorter survival in patients who had an initially rapid response to therapy or a lesser reduction in serum M-protein concentration.14
Berenson et al. conducted a study to evaluate the efficacy of single-agent corticosteroid maintenance. They randomized 126 patients who achieved a response after induction therapy to either physiologic dose prednisone (10 mg) or pharmacologic (50 mg) doses of alternate day oral prednisone until disease progression. With a median follow-up of 44 months, PFS was significantly improved in the high-dose prednisone arm compared with the low-dose prednisone arm (14 versus 5 months). Median OS was also significantly prolonged in the high-dose group compared with the lowdose group (37 versus 26 months). There was no difference in treatment related adverse events between arms.15 Shustik et al. also evaluated the efficacy of single-agent dexamethasone maintenance therapy. They randomized 585 transplant-ineligible patients to either dexamethasone maintenance or observation after melphalan and dexamethasone or MP induction therapy. Dexamethasone maintenance has prolonged PFS significantly (2.8 versus 2.1 years), but no OS benefit was observed in the maintenance group (4.1 versus 3.8 years).16
Given the lack of sufficient evidence to support the use of single agent or combination chemotherapies as maintenance therapy and high toxicity rates, investigators then evaluated less-toxic approaches as maintenance therapy. MM was the first malignant disease shown to be sensitive to a cytokine, IFN, leading to its popularity as a maintenance approach. Several clinical trials were performed evaluating the possible role of IFN.17 Fritz et al. reported a meta-analysis of all available published data including 13 trials with 1,615 patients, evaluating IFN as a potential maintenance strategy. IFN maintenance therapy led to 4.4- and 7.0-month prolongations of relapse-free survival and OS, respectively. They concluded that the results of the meta-analysis favored the use of IFN maintenance treatment, which resulted in considerable survival gains at economically feasible costs.18 The Myeloma Trialists’ Collaborative Group subsequently performed another meta-analysis, which evaluated the benefits of IFN as an induction and maintenance therapy, including 24 trials and 4,012 patients.19 During induction, response rates were slightly better with IFN (57.5 % versus 53.1 %). PFS was better with IFN (33 % versus 24 % at 3 years), an effect seen in both induction and maintenance portions. Median time to progression was increased by about 6 months in both settings. OS was also better with IFN (53 % versus 49 % at 3 years) with median survival increased by about 4 months. This benefit was, however, restricted to the smaller trials. The effect of IFN was not significantly related to dose or duration of IFN or to patients’ characteristics. While PFS was improved with IFN, the survival benefit when seen was small and needed to be balanced with cost and toxicity.
In conclusion, long-term use of these agents was limited by toxicity and modest efficacy. The available evidence was not sufficient to recommend any of these historical strategies routinely as a maintenance approach.
Maintenance Approaches with Novel Agents
Introduction of novel therapies, including proteasome inhibitors, bortezomib, and, later, carfilzomib and the immunomodulatory agents, thalidomide, lenalidomide, and, most recently, pomalidomide, have dramatically improved OS of patients with MM. The significant efficacy of these drugs in the newly diagnosed setting, as well as in the relapsed setting, with manageable toxicities has paved the way for maintenance trials with these drugs.
Thalidomide
Thalidomide was the first immunomodulatory agent that showed efficacy in the treatment of MM.20,21 It has been studied as a maintenance therapy in clinical trials, either as a single agent or combined with corticosteroids or other agents with varying efficacy (see Table 1). Attal et al. conducted a randomized trial of maintenance treatment with thalidomide and pamidronate. In the IFM trial (IFM 99-02), 2 months after high-dose therapy, 597 patients younger than 65 years old were randomly assigned to receive no maintenance (arm A), pamidronate (arm B), or pamidronate plus thalidomide (arm C). Overall, 55 % of patients in arm A, 57 % in arm B, and 67 % in arm C achieved a complete or very good partial response. Both 3-year event-free survival and 4-year OS rates were significantly higher in the thalidomide maintenance group compared with other two groups.22
Barlogie et al. randomized 668 patients to receive thalidomide until disease progression or unacceptable side effects. After a median follow-up of 42 months the thalidomide and control groups had 5-year event-free survival rates of 56 % and 44 %. The 5-year rate of OS was approximately similar (65 %) in both groups. Median survival after relapse was 1.1 years in the thalidomide group and 2.7 years in the control group. Severe peripheral neuropathy and deep-vein thrombosis occurred more frequently in the thalidomide group than in the control group.23 Long-term follow-up of this trial showed a segregation in survival plots in favor of thalidomide maintenance after 5 years of follow-up, but a statistically significant improvement of OS was observed in patients possessing adverse cytogenetic abnormalities.24
In 2012, Morgan et al. reported the results of MRC IX trial and a metaanalysis of that and previous studies to evaluate the efficacy of thalidomide maintenance. In the MRC IX trial, after intensive or nonintensive induction therapy, 820 newly diagnosed MM patients were randomized to open-label thalidomide maintenance until progression, or no maintenance. Interphase fluorescence in situ hybridization (iFISH) analysis was performed at study entry. Median PFS was significantly longer with thalidomide maintenance, but median OS was similar between regimens. Patients with favorable iFISH showed improved PFS and a trend toward a late survival benefit. By contrast, those with adverse iFISH receiving thalidomide showed no significant PFS benefit and ‘worse’ OS. Extended follow-up (5.9 years) results of MRC IX showed that thalidomide maintenance improved PFS but not OS, and was associated with significantly shorter OS in patients with adverse cytogenetics (p=0.01).25,26
In 2010, Lokhorst et al. reported the HOVON-50 trial, which was designed to evaluate the effect of thalidomide during induction treatment and as maintenance in patients with MM who were transplant candidates. A total of 556 patients was randomly assigned to arm A: three cycles of vincristine, adriamycin, and dexamethasone (VAD), or arm B: thalidomide 200 mg orally, continuously, plus adriamycin and dexamethasone (TAD). After induction therapy and stem cell mobilization, patients received high-dose melphalan, 200 mg/m2, followed by maintenance with alpha-IFN or thalidomide 50 mg daily. Thalidomide significantly improved the overall response rate, as well as the quality of the response before and after high-dose melphalan. Thalidomide also significantly prolonged PFS from median 25 months to 34 months. Median OS was longer in the thalidomide arm, although not statistically significant (73 versus 60 months). Despite longer PFS, patients randomized to thalidomide had reduced survival after relapse.27
Stewart et al. reported a randomized, controlled trial comparing thalidomide-prednisone as maintenance therapy with observation in 332 patients who had undergone autologous stem cell transplantation with melphalan 200 mg/m2 (MY 10 trial). The primary endpoint was OS; secondary endpoints were myeloma-specific PFS, PFS, incidence of venous thromboembolism, and health-related quality of life (HRQoL). With a median follow-up of 4.1 years, no differences in OS between thalidomideprednisone and observation were detected; thalidomide-prednisone was associated with superior myeloma-specific PFS and PFS (4-year estimates were 32 % versus 14 %) and more frequent venous thromboembolism (7.3 % versus none). Those allocated to thalidomide-prednisone reported worse HRQoL with respect to cognitive function, dyspnea, constipation, thirst, leg swelling, numbness, dry mouth, and balance problems.28
Spencer et al. examined whether the addition of 12 months thalidomide consolidation following AHSCT would improve the durability of responses achieved and OS. Post-AHSCT, 129 patients were randomly assigned to receive indefinite prednisolone maintenance therapy (control group) and 114 to receive the same in addition to 12 months of thalidomide consolidation (thalidomide group). After a median follow-up of 3 years, 3-year PFS rates were 42 % and 23 % and the OS rates were 86 % and 75 % in the thalidomide and control groups, respectively. There was no difference in survival between groups 12 months after disease progression (79 % versus 77 %). Neurologic toxicities were more common in the thalidomide arm, but there were no differences between arms for thromboembolic events.29
Krishnan et al. integrated thalidomide maintenance to their randomized trial, comparing the effectiveness of allogeneic HSCT with nonmyeloablative conditioning after AHSCT with tandem AHSCT. In the auto-auto group 217 patients received maintenance treatment, 77 % of who completed planned maintenance. PFS and OS did not differ between maintenance and observation groups.30
Maiolino et al. examined the efficacy of thalidomide plus dexamethasone as a maintenance therapy after AHSCT. One hundred and eight patients were randomized to receive maintenance with dexamethasone or dexamethasone with thalidomide (200 mg daily) for 12 months or until disease progression. After a median follow-up of 27 months, 2-year PFS was 30 % in the dexamethasone arm and 64 % in the thalidomide and dexamethasone arm. OS at 2 years was not significantly improved with the addition of thalidomide (70 % versus 85 % respectively).31
Authors of MRC IX trial have performed a meta-analysis of their and other studies, which included thalidomide-maintenance therapy. They showed a significant late OS benefit. Thalidomide maintenance consistently improved PFS but with variable effects on improved OS.26 Kagoya et al. performed another meta-analysis, which included six trials and 2,786 patients to assess the efficacy of thalidomide maintenance. Patients treated with thalidomide maintenance did not have significantly better OS. The improvement was especially prominent in a subgroup of studies using corticosteroids with thalidomide. Thalidomide improved PFS, but had more frequent venous thrombosis and peripheral neuropathy. The results of this meta-analysis suggested that thalidomide maintenance with corticosteroids is effective in prolonging survival for MM.32
In conclusion, thalidomide maintenance certainly prolonged PFS but had a variable impact on OS. The variability in OS benefit likely reflects the heterogeneity among the studies and the availability of effective salvage therapies. While prolonging PFS, in one study it significantly diminished patient-reported health-related quality of life. An important thing to emphasize is that ‘worse’ OS was documented in some trials among the high-risk patients.
Bortezomib
Bortezomib is a proteasome inhibitor, which has efficacy in treatment of both newly diagnosed and relapsed MM.33,34 It has been studied as a maintenance therapy in clinical trials, either as a single agent in combination with corticosteroids or other agents with varying efficacy (see Table 2).
Bortezomib was evaluated as a maintenance therapy after AHSCT in the HOVON-65/GMMG-HD4 trial, where 827 eligible patients with newly diagnosed symptomatic MM were randomly assigned to receive induction therapy with VAD or bortezomib, doxorubicin, and dexamethasone (PAD), followed by high-dose melphalan and AHSCT. Maintenance consisted of thalidomide 50 mg after VAD induction once per day or bortezomib 1.3 mg/m2 after PAD induction biweekly for 2 years. Complete response rate (CR) was superior after PAD induction (15 % v 31 %) and bortezomib maintenance (34 % v 49 %). After a median follow-up of 41 months, PFS was superior in the PAD arm (median of 28 versus 35 months). In multivariate analysis, OS was better in the PAD arm. In high-risk patients presenting with increased creatinine more than 2 mg/dL, bortezomib significantly improved PFS from a median of 13 months to 30 months and OS from a median of 21 months to 54 months. A benefit was also observed in patients with deletion 17p13 (median PFS, 12 versus 22 months; median OS, 24 months versus not reached at 54 months). In the thalidomide arm, 64 % of the patients discontinued maintenance therapy because of progressive disease (PD), toxicity, and other reasons (31 %, 31 %, and 2 %, respectively). In the bortezomib arm, 47% discontinued maintenance therapy because of PD, toxicity, and other reasons (29 %, 9 %, and 9 %, respectively). Grade 3–4 PNP rate was significantly greater in the PAD group (16 % versus 7 %).35 However, given the study design, it is hard to differentiate if the benefit came from the introduction of bortezomib as part of induction or maintenance or both.
Rosinol et al. reported a PETHEMA group phase III randomized trial examining three induction regimens: the triple combination bortezomib, thalidomide, and dexamethasone (VTD), thalidomide and dexamethasone (TD), and the alternating combination chemotherapy of vincristine, carmustine, melphalan, cyclophosphamide, prednisone/vincristine, carmustine, doxorubicin, dexamethasone. After induction, patients underwent a melphalan-based single AHSCT with a randomization to maintenance therapy for three years with bortezomib plus thalidomide (VT), thalidomide alone or IFN-α alone. At a median follow-up of 24 months from the initiation of maintenance, the VT arm had a significantly longer PFS than thalidomide or IFN-a (78 % versus 63 % versus 49 %). There was no OS benefit seen across the three maintenance arms.36
Bortezomib has also been examined in the non-transplant setting. Mateos et al. compared two different bortezomib-based maintenance treatments for transplant-ineligible patients in the GEM2005MA trial. One hundred and seventy eight transplant-ineligible patients were randomized to either bortezomib plus prednisone or bortezomib plus thalidomide maintenance treatments after six cycles of induction therapies upfront with either melphalan, bortezomib, plus prednisone (VMP) or melphalan, thalidomide, plus prednisone (VTP). Maintenance consisted of one conventional cycle of bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11) every 3 months, plus either oral prednisone 50 mg every other day or oral thalidomide 50 mg per day up to 3 years. After a median follow-up of 46 months, the CR rate increased from 24 % at the end of induction (mean CR rate obtained after VMP and VTP) to 42 %. Although there were no significant differences between both maintenance arms, the CR rate was slightly higher for VT compared with VP (46 % versus 39 %, p=not significant [NS]). Median PFS was superior for patients randomized to VT compared with patients who received VP (39 versus 32 months), although this difference was NS (p=0.1). OS was also slightly longer in patients maintained with VT compared with VP (5 year OS of 69 versus 50 %; p=0.1).37
Palumbo et al. reported the GIMEMA trial, which was designed to compare VMP versus VMP plus thalidomide (VMPT) as induction therapy for transplant- ineligible patients. After induction with VMPT, patients were randomized to maintenance treatment with VT, including bortezomib 1.3 mg/m2 bi-weekly and thalidomide 50 mg daily for 2 years or until disease progression, while no maintenance was given to patients in the VMP arm. Patients receiving VMPT and VT maintenance had a higher CR rate (24 % versus 38 %, at 3 years), longer PFS (56 % versus 41 %, at 5 years) and OS (59.3 % versus 45.9 %, at 5 years). The median duration of VT maintenance was 23.8 months. During VT maintenance 7 % of patients experienced grade 3–4 peripheral neuropathy and 12 % discontinued due to adverse events.38,39
Lenalidomide
Lenalidomide was the second immunomodulatory drug, which showed efficacy for the treatment of both newly diagnosed and relapsed myeloma.40,41 It has a stronger antimyeloma effect and a more favorable toxicity profile compared with thalidomide.1 It is considered a feasible maintenance treatment because it is an oral agent, has efficacy in low doses, and, as a single agent, can be given orally and has a well-defined toxicity profile. Efficacy of lenalidomide as a maintenance therapy was evaluated in phase III, double-blind, randomized trials, which included both transplant-eligible and ineligible patients (see Table 3).
In the IFM 05-02 study, patients having received previous induction therapy with either vincristine, doxorubicin, plus dexamethasone or bortezomib plus dexamethasone (VD) followed by one or two AHSCT were treated with two cycles of lenalidomide consolidation therapy and were, thereafter, randomized to lenalidomide maintenance (LM) (10–15 mg daily) or placebo until disease progression. After a median follow-up of 45 months from randomization, the 4-year estimates of PFS were 43 % for the lenalidomide group and 22 % for the placebo group, while no difference in OS was seen between the two groups (73 % versus 75 %). The study was unblinded at median follow-up of 32 months and all maintenance was stopped at a median time of 32 months for the lenalidomide arm due to concern regarding the development of second primary malignancy (SPM). The placebo group patients did not crossover to receive lenalidomide at unblinding. The lenalidomide group had an increased incidence of hematologic toxicities (primarily neutropenia) and an increase in SPMs (hematologic malignancies) of 13 versus five patients and solid organ malignancies of 10 versus four patients for lenalidomide and no maintenance groups consecutively. The median EFS were 40 months for the lenalidomide group and 23 months for the placebo group. With a longer follow-up of 60 months, LM improved PFS (42 %) compared with placebo (18 %). OS was similar in both groups (68 % at 5 years for the lenalidomide group versus 67 % for the placebo group) possibly as a result of the significantly shorter time from first to second relapse/progression observed for patients randomized to LM (10 versus 18 months for the placebo group).42,43
The Cancer and Leukemia Group B (CALGB) designed a similar study (CALGB 100104) for patients treated with one AHSCT following induction therapy. They randomly assigned 614 patients younger than 65 years of age who had no progressive disease after first-line transplantation to maintenance treatment with either lenalidomide (10 mg per day for the first 3 months, increased to 15 mg if tolerated) or placebo until relapse. The primary endpoint was PFS. The induction regimens varied with 74 % of patients receiving a thalidomide- or lenalidomidebased induction regimen. The median time to progression (TTP) was 46 months for the lenalidomide group and 27 months for the placebo group. The 3-year PFS was 66 % for the lenalidomide group and 39 % for the placebo group. At a median follow-up of 34 months, the 3-year OS rates were 88 % for the lenalidomide group and 80 % for the placebo group. The study was updated in 2013 with a median follow-up of 48 months, and the OS was 80 % for the lenalidomide group and 70 % for the placebo group. The study had been unblinded at a median follow-up of 26 months because the primary endpoint (TTP) had been met and 67 % of eligible placebo patients crossed over to receive lenalidomide. A TTP and OS benefit for the lenalidomide arm remained despite the crossover. The lenalidomide group had an increased incidence of hematologic toxicities (primarily neutropenia) and an increase in SPMs. There were eight (3.5 %) hematologic malignancies, primarily acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) in lenalidomide group patients versus one (0.4 %) in placebo group patients. There were 10 (4.3 %) versus five (2.1 %) solid tumors in lenalidomide group and placebo group patients, respectively. In the 2013 update, one AML was reported in the lenalidomide arm (total nine of 231 hematologic malignancies) and one case of acute lymphoblastic leukemia and MDS in two placebo arm patients who crossed over to lenalidomide. The cumulative incidence risk for the development of SPM was greater for the lenalidomide group compared with the placebo group. PFS and OS were significantly longer at the LM group. The study showed that although LM therapy was associated with more toxicity and SPMs, the patients benefited from significantly longer PFS and improved OS.44,45
Palumbo et al. have published their data regarding the AHSCT and maintenance therapy in MM. In a phase III study, they compared high-dose melphalan plus AHSCT with melphalan, prednisolone plus lenalidomide (MPR) and maintenance therapy with no maintenance therapy. Primary endpoint of the study was PFS. Here, 273 newly diagnosed transplant-eligible patients were randomized to either highdose melphalan plus stem cell transplantation or MPR after initial induction therapy with lenalidomide plus dexamethasone. After stem cell transplantation or MPR consolidation, 251 of 273 patients were rerandomized to either maintenance or no maintenance. Maintenance therapy with lenalidomide (10 mg on days 1–21 of each 28-day cycle) was administered until disease progression or development of unacceptable adverse effects. Among these 251 patients, median PFS was significantly longer with LM therapy than with no maintenance therapy (41.9 versus 21.6 months). LM therapy compared with no maintenance therapy had no significant effect on the 3-year OS rate (88 % versus 79.2 %). The beneficial effect of maintenance therapy on PFS was similar in both stem cell transplantation and MPR consolidation arms. Eleven patients (2.8 %) had a second primary cancer in various phases of study. Three of these 11 patients developed second primary cancer during the LM therapy.46
LM was also assessed in the setting of transplant-ineligible patients. The MM-015 study randomized elderly patients not eligible for AHSCT to receive either MP or MPR, both given for nine cycles, followed by placebo, or MPR for nine cycles, followed by LM (MPR-R) until disease progression. PFS was similar for the MP and MPR arm, but was significantly longer for patients receiving MPR-R (13 months versus 14 months versus 31 months, respectively). A landmark analysis from starting maintenance in patients receiving MPR or MPR-R demonstrated that LM significantly extended PFS compared with placebo (26 months versus 7 months), although no OS benefit could be demonstrated.47
Benboubker et al. have published the results of FIRST trial. The trial was primarily designed to assess the efficacy of lenalidomide and low-dose dexamethasone therapy in transplant-ineligible patients. One thousand six hundred and twenty-three patients were randomly assigned to lenalidomide and dexamethasone in 28 day cycles until disease progression, to the same combination for 72 weeks (18 cycles), or to melphalan, prednisone, plus thalidomide (MPT) for 72 weeks. Despite maintenance therapy not being included in the design of the study, using continuous lenalidomide and low dexamethasone as a separate therapy arm can represent a maintenance-like approach. A total of 39 % of the patients assigned to continuous lenalidomidedexamethasone received more than 2 years of study treatment. With a median follow-up of 37 months, the median PFS was 25.5 months with continuous lenalidomide–dexamethasone, 20.7 months with 18 cycles of lenalidomide–dexamethasone, and 21.2 months with MPT. Continuous lenalidomide–dexamethasone was associated with a significant improvement in PFS compared with MPT (p<0.001). Continuous lenalidomide–dexamethasone also reduced the risk for progression or death compared with 18 cycles of lenalidomide plus dexamethasone (p<0.001). Four-year OS rates were 59 %, 56 %, and 51 % consecutively for continuous lenalidomide, 18 cycles of lenalidomide, and MPT arms, respectively. Continuous lenalidomide and dexamethasone therapy significantly improved OS compared with MPT (p=0.02). Invasive second primary cancers were reported in 17 patients (3 %) who received continuous lenalidomide–dexamethasone, in 30 (6 %) who received 18 cycles of lenalidomide–dexamethasone, and in 27 (5 %) who received MPT. Hematologic cancers were more frequent with MPT.48
A meta-analysis of these lenalidomide studies including 1,935 patients has since been conducted. There was significant prolongation of both PFS (hazard ratio [HR] 0.49, 95 % confidence interval [CI], 0.41–0.58; p<0.001) and OS with lenalidomide versus placebo/no maintenance. Best response during maintenance was reported only in two studies and odds of very good partial response or better were not significantly different with maintenance. There was a nearly twofold increase in the risk for SPMs with LM. Meta-analysis of RCTs demonstrated significant improvement in PFS and modest improvement in OS with LM. There was an increased risk for grade 3–4 adverse effects, including SPMs with LM. Substantial heterogeneity for estimate of OS among protocols was considered as a limitation of this analysis.49
Summary and Recommendations
The landscape of myeloma treatments continues to evolve rapidly with the introduction of new drugs. The question of maintenance is entwined with the question as to the ideal duration of therapy. From our experience so far, we realize that myeloma continues to relapse after the most aggressive treatment approaches. Given this background, the interest in continuing to intensify treatment of the disease is clearly understandable. Addition of more drugs to the current regimens do not necessarily translate into more benefit as was shown with the bortezomib, dexamethasone, cyclophosphamide, and lenalidomide (CRVD) and bortezomib, dexamethasone, cyclophosphamide, and thalidomide (CTVD) combinations in clinical trials. Instead of intensifying the regimen, an alternate approach would be to give a more tolerated combination for a longer time, still achieving the goal of maximal tumor reduction. In the patient undergoing an autologous stem cell transplant, this prolonged course of treatment is considered a maintenance, whereas in the nontransplanted patients, it represents continued therapy, bringing up the question of ideal therapy duration.
Given the seemingly contradictory data, definitive conclusions remain difficult to make. However, taking into account the benefit shown in the US phase III trial among those not achieving a CR/very good partial response (VGPR), it is reasonable to consider offering maintenance (aka prolonged therapy). Such patients can certainly be considered for LM. However, in the high-risk patients prolonged treatment with bortezomibbased regimens appear to be justified based on the HOVON data examining the role of PAD. We recommend putting patients with highrisk myeloma on a bortezomib maintenance. For the remaining patients, one could consider a short consolidation as shown in the French trial: namely, two cycles of lenalidomide–dexamethasone with no continued therapy, given that this trial failed to show any difference in OS. Clearly, newer trials such as the one using oral proteasome inhibitor ixazomib would be of great interest.