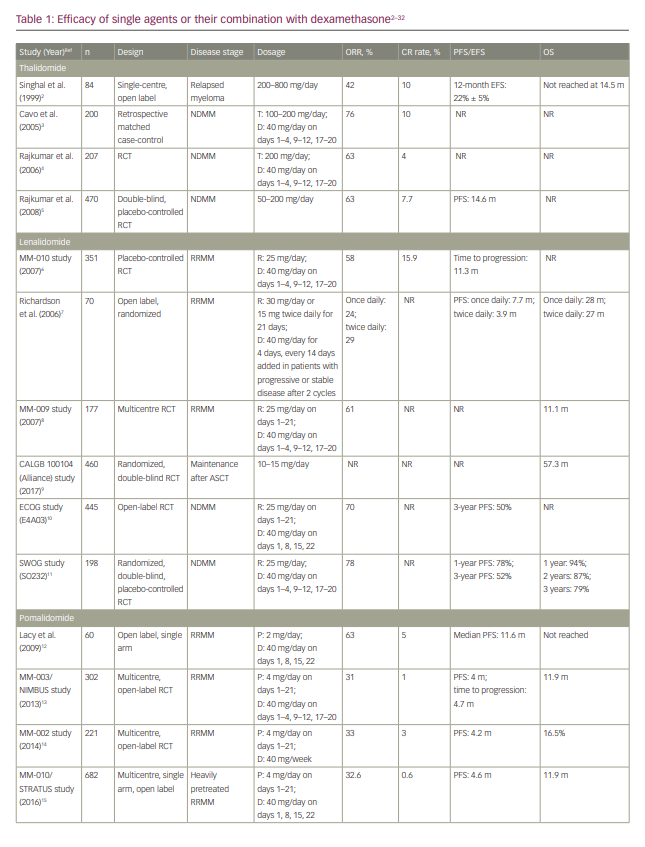
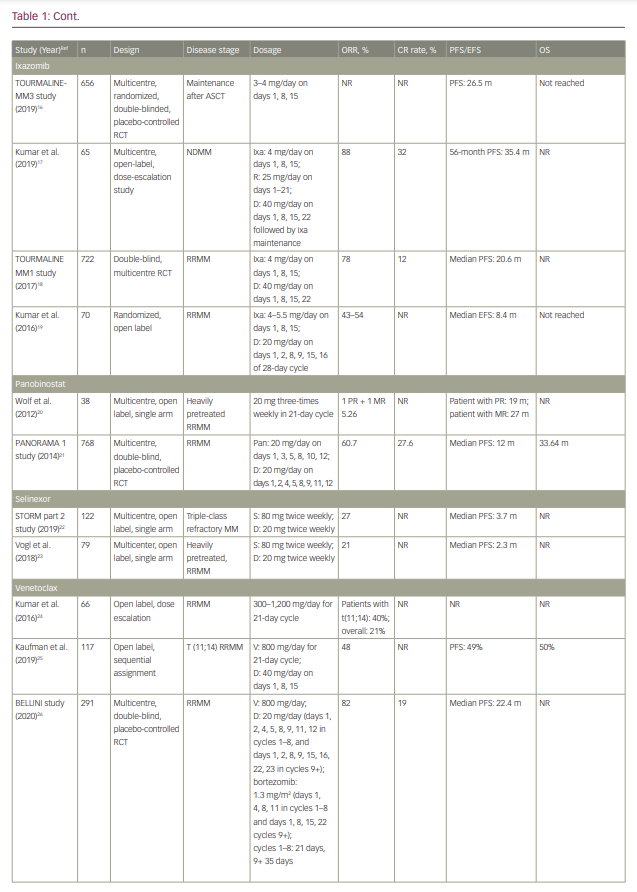
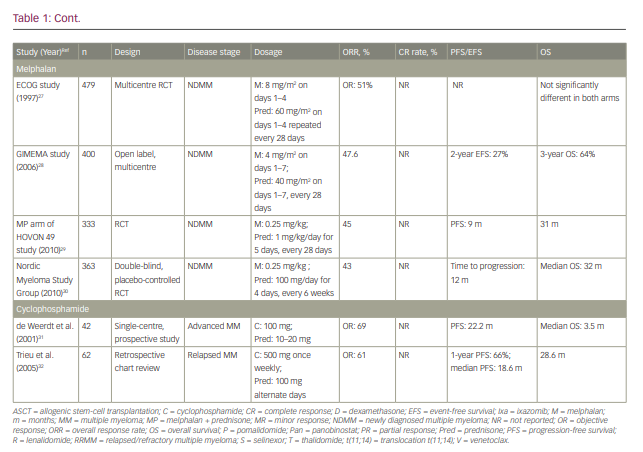
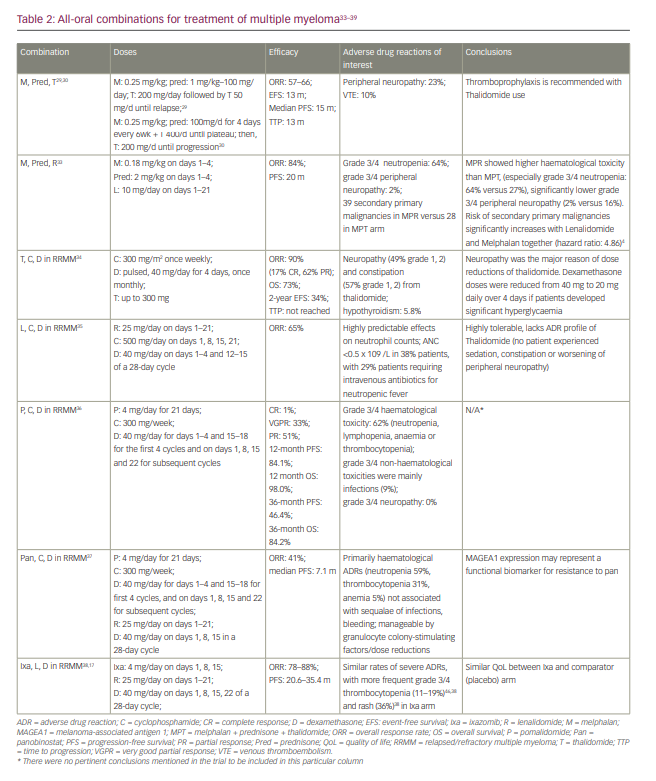
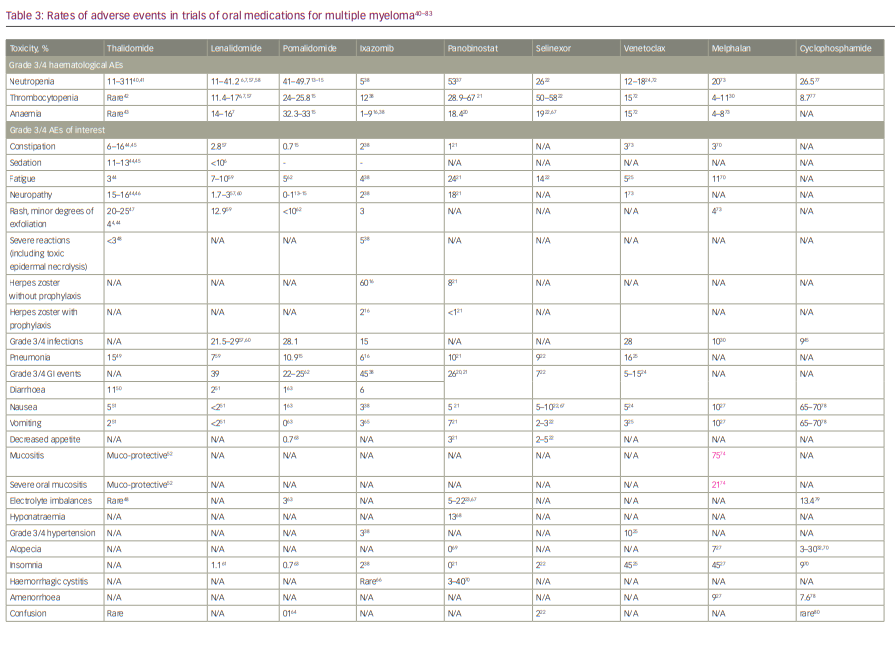
Treatment options for myeloma have rapidly expanded in the past decade and, as with many cancers, the number of oral drug options has increased considerably. Given the more limited number of cancer hospitals, patients often must travel considerable distance to get to their closest cancer centre, which places a significant burden on receiving adequate therapy. While most historical cancer therapies were parenteral, increasing availability of oral drugs has reduced the need for frequent hospital visits. Fewer hospital visits can improve quality of life, reduce out-of-pocket travel expenses, which are often not reimbursed by healthcare plans, and potentially reduce risk of infections.1 This review focuses on the currently available oral therapies for myeloma, their efficacy and safety, and the different combinations that have been studied in clinical trials. We discuss oral therapies for multiple myeloma (MM), including immunomodulatory drugs (IMiDs) thalidomide, lenalidomide and pomalidomide; the proteosome inhibitor ixazomib; the histone deacetylase inhibitor panobinostat; and newly available drugs selinexor and venetoclax. The historical standard of care, cyclophosphamide and melphalan-based therapies, and their currently used regimens are also discussed, followed by current data from trials on each drug alone or with dexamethasone. Finally, all-oral combination regimens of these therapies are summarized. Table 1 shows the efficacy of therapies taken alone or in combination with dexamethasone.2–32 Table 2 shows all the oral combinations for treating MM.33–39 Table 3 displays the rates of adverse events (AEs) in the trials of oral medications for MM.40–83
Immunomodulatory drugs
Thalidomide was the first drug in this class and was introduced for treatment of myeloma in 1999.84 Since then, it has been studied in various combinations in different disease stages. Thalidomide was followed by lenalidomide and pomalidomide, with all three differing in efficacy and toxicity profiles.85 This class of drugs remains the backbone of many of the regimens currently used in the clinic.
Thalidomide
Clinical efficacy
Thalidomide has been used alone,86 but most of the efficacy data relate to steroid combinations, as is the case with most of the myeloma therapies.49,87,88 The response rates to thalidomide and dexamethasone in newly diagnosed and relapsed disease are shown in Table 1.
Dose and schedule
Thalidomide has been used at doses ranging from 50 mg daily to 800 mg daily, but most patients will not tolerate doses above 200 mg/day, with the drug given without a break. In most situations, it can be started at 100 mg daily, and then escalated if needed to 200 mg daily. It can be combined with 40 mg weekly of dexamethasone.40
Side effects
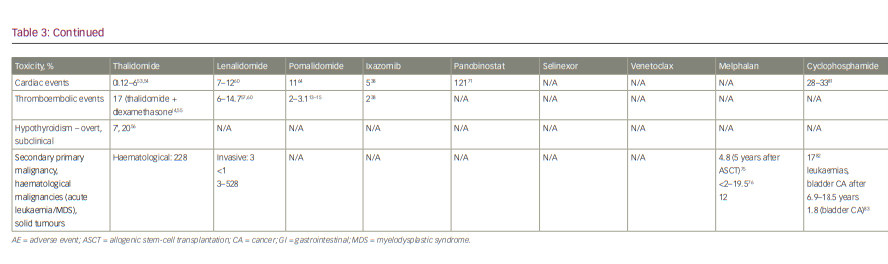
Somnolence, neuropathy and constipation are the most common adverse effects associated with thalidomide, and have been uniformly observed in all studies. Patients often become tolerant to some of these effects, such as the somnolence and constipation; however, other effects, such as the neuropathy, can be progressive if the drug is not discontinued or its dose reduced. Other less common side effects include bradycardia, venous thromboembolism, severe skin reactions such as toxic epidermal necrolysis, thyroid dysfunction and elevated liver enzymes.40 The safety profile of thalidomide has been summarized in Table 3.
Venous thrombosis: Nearly 10% of patients with MM develop deep vein thrombosis (DVT) when treated with chemotherapy alone; however, the incidence is much higher when chemotherapy is combined with thalidomide (28%).89 The risk was highest in patients with acquired protein C resistance on thalidomide, in absence of factor V Leiden mutation (50%).89 Patients treated with thalidomide at first line are generally at higher risk than those in the relapsed/refractory setting.43 Patients treated with thalidomide also have an increased risk of arterial thromboembolism, including myocardial infarctions and cerebrovascular accidents.90 Also of note, concurrent use of oral contraceptives with thalidomide/dexamethasone combination can have additive effects on risk of thromboembolism, and contraception should be picked carefully before initiation of this therapy.40
Thromboprophylaxis is not routinely recommended for patients receiving single-agent or maintenance thalidomide, but is strongly recommended for patients receiving thalidomide with high-dose dexamethasone or chemotherapy.42 The International Myeloma Working Group guidelines specifically address thromboembolism in patients with myeloma treated with IMiDs, and recommend thromboprophylaxis especially for patients taking IMiDs in combinations.91
Venous thrombosis (VTE) risk factors can be classified into three categories: patient-related, disease-related or treatment-related. Most guidelines recommend aspirin in patients with no or one risk factor, while those with two or more risk factors should be placed on low-molecular-weight heparin (LMWH) or full-dose warfarin.43,91–93 However, other novel options like direct oral anti-coagulants, such as apixaban, are an attractive option, as they are administered orally with fixed daily dosing, which is much more convenient than the subcutaneous dosing of LMWH and the frequent monitoring required with warfarin therapy.94
Dermatological manifestations: Although rashes and minor exfoliation are seen in 20–25% of patients with myeloma receiving thalidomide, toxic epidermal necrolysis has been reported only rarely.95 Patients taking thalidomide in combination with dexamethasone had a higher incidence of severe reactions than those taking thalidomide alone (4% versus 19%, respectively).44 Most skin rashes occurred during the first month of treatment; however, late-onset rashes were reported up to 4 months after starting therapy.44 Patients who develop a rash should have their treatment held, then reinstated at a lower dose once the rash subsides. If the rash recurs, then the drug should be discontinued.
Hypothyroidism: Subclinical hypothyroidism occurred commonly in patients with MM who were treated with thalidomide.56,96 Consequently, it may contribute to the adverse effects seen with thalidomide, including fatigue, constipation, neuropathy and bradycardia.96 While overt hypothyroidism is uncommon, early symptoms are often overlooked as thalidomide itself also causes fatigue.96 Checking thyroid-stimulating hormone (TSH) levels every 3–6 months in patients receiving thalidomide, and providing thyroid hormone replacement if this complication occurs is prudent when using thalidomide in patients with MM.
Teratogenicity: Teratogenicity is the most publicized adverse effect of thalidomide. It causes phocomelia and is frequently associated with malformations of inner organs.97 Mortality at birth or shortly after has been reported in about 40% of infants whose mother was treated with thalidomide.97,98 Therefore, pregnancy is an absolute contraindication, and a negative pregnancy test is required before starting IMiD therapy in women of childbearing potential. Contraception should be maintained throughout the duration of therapy.
Somnolence: Somnolence during thalidomide treatment is common, and patients should be warned about driving or operating heavy machinery. An evening dosing strategy minimizes somnolence, and the effect reduces with use.2
Constipation: Constipation is another common side effect of thalidomide, and pre-emptive laxatives are highly recommended.2
Peripheral neuropathy: This manifests mainly as sensory neuropathy, or neuropathic pain, and is one of the most commonly seen side effects with thalidomide. A complete neurological exam is imperative on each clinic visit to screen for signs of peripheral neuropathies. Dose reductions can be considered if symptoms worsen when peripheral neuropathy is mild grade (1 or 2). Dose interruptions are also a preferable line of management in cases of grade 2 peripheral neuropathy. The patient can be rechallenged with thalidomide after assessing an individual risk/benefit ratio. Grade 3 and 4 neuropathy warrant complete discontinuation of thalidomide. All cases of neuropathy are not reversible.40,87
Monitoring for patients on thalidomide includes:87
- Complete blood count,
- Signs and symptoms of DVT/pulmonary embolism (PE): dyspnoea,
leg swelling, chest pain, - International normalized ratio if patient is on warfarin for thromboprophylaxis,
- TSH levels every 3–6 months,
- Negative pregnancy test before initiating therapy in women of childbearing potential,
- Complete neurological exam on each visit to monitor for peripheral neuropathy.
Lenalidomide
Lenalidomide is the second drug of this class to be approved for use in patients with myeloma. It is currently used alone as maintenance therapy after autologous stem-cell transplant (ASCT) in patients with standard-risk, becoming a new benchmark for survival.99 Most of its efficacy data come from combinations with dexamethasone in both newly diagnosed patients and patients with relapsed MM, as shown in Table 1.
Dose and schedule64
Lenalidomide has been used in doses ranging from 10 mg daily to 25 mg daily, though most common is 25 mg daily for 21 days every 28 days in patients with myeloma.64 For maintenance, 10–15 mg/day is used until progression.99 It is typically combined with dexamethasone 40 mg weekly. In this combination, it is active and well tolerated in patients with newly diagnosed MM.95 It is the backbone of many triplet therapies, as given in Table 2.
Side effects
While chemically related to thalidomide, lenalidomide’s efficacy and toxicity profile differ significantly from thalidomide. Although it produces a similar or higher rate of cytopenias than thalidomide, the risk of neuropathy is much lower.44,46,57,60 The safety profile of lenalidomide has been summarized in Table 3. Severe adverse drug reactions are rare,
if any occur.44,57,87
Cytopenias: Lenalidomide-induced neutropenia should always be considered, because neutropenia is one of the most frequent side effects of lenalidomide.57 If absolute neutrophil count (ANC) decreases to <1,000/mm3, lenalidomide should be interrupted and ANC monitored weekly. Upon recovery, lenalidomide can be resumed at the previous dose if neutropenia is the only toxicity, or at the next lowest dose if there are other toxicities. For each subsequent decrease to <1,000/mm3, lenalidomide should be interrupted and resumed at the next lowest dose upon recovery.64 Dose interruptions or reductions are needed if platelets fall below 30,000/mm3. Platelet transfusions are rarely required and reserved for those with bleeding.64 Management with dose reductions or interruptions may be needed, and in some cases, use of granulocyte colony-stimulating factor (G-CSF) may
be necessary.100,101
VTE: Although slightly lower than thalidomide, the risk of VTE with lenalidomide use in patients with MM is significant, especially with dexamethasone and alkylating agents.57,60 It is recommended to continue patients on lenalidomide on thromboprophylaxis (with LMWH or adjusted-dose warfarin) or daily aspirin, as per risk stratification of the individual patient.50,59,91
Teratogenicity: Much like thalidomide, lenalidomide is also a drug of high teratogenic potential, and is contraindicated in women of childbearing potential without effective contraception and thorough discussion prior to initiation of drug.64
Monitoring for patients on lenalidomide includes:64
- Regular blood counts should be performed: weekly for first 2–3 cycles, and every 4 weeks thereafter once the trends are stable.
- Patients with neutropenia should be monitored for signs of infection, and those with thrombocytopenias for signs of bleeding, epistaxis, mucocutaneouspetechiae, etc. See above for dose changes for the same.
- Signs and symptoms of DVT/PE are the following: dyspnoea, leg swelling, chest pain.
- Pregnancy testing: obtain two negative pregnancy tests before starting lenalidomide – one 10–14 days prior to starting and the second 24 hours prior to starting. Pregnancy tests should be performed monthly thereafter in women with regular menstrual cycles and every 2 weeks in women with irregular menstrual cycles.102
Pomalidomide
Pomalidomide is the third IMiD to be introduced, which, in combination with dexamethasone, is active in patients with relapsed/refractory MM.85 Pomalidomide with low-dose dexamethasone can be used in lenalidomide- and bortezomib-refractory myeloma, and is well tolerated.102 It has a much more potent anti-myeloma effect than thalidomide or lenalidomide, with a different safety profile.85
Dose and schedule
Pomalidomide has been used at doses ranging from 2 mg daily to 4 mg daily in patients with relapsed/refractory MM. The recommended starting dose is 4 mg daily for 21 days of a 28-day cycle, but 1 mg, 2 mg and 3 mg doses are available for dose modification.103 It can be combined with dexamethasone 40 mg weekly.11
Side effects
Given the high disease burden, residual effects of prior treatments and comorbidities, patients with relapsed/refractory MM are susceptible to treatment-related AEs.104 In clinical trials, the rates of DVT and PE were low due to patients receiving thromboprophylaxis (2.0–3.1%);13–15 but other treatment‐emergent AEs most commonly leading to dose reductions of pomalidomide are neutropenia, thrombocytopenia and infections.15 Its safety profile is highly favourable, with mainly haematological toxicities that can be easily managed.105 The adverse drug reactions are summarized in Table 3.
Cytopenias: Depending on its severity, neutropenia can be managed with dose interruptions, dose modifications and/or growth-factor support. granulocyte colony-stimulating factor therapy can be administered to patients experiencing neutropenia and febrile neutropenia. Anti-infective agents can be used in patients with infections or febrile neutropenia.62,106 Thrombocytopenia is managed with dose interruptions if platelets fall below 25,000/mm3. Upon recovery of platelets, dosing should be resumed at the next lowest dose. For each subsequent drop to <30,000/mm3, pomalidomide should be interrupted and resumed at 1 mg less than previous dose upon recovery.103 Dose interruptions are also recommended if ANC drops below 500/mm3.103
Infections: Antibiotic prophylaxis can be considered for patients receiving pomalidomide, especially in the first months after starting treatment, when risk of infection is highest.107 For patients with very high risk of infection (low blood counts, history of infection or both), consider prophylaxis for the entire duration of therapy.62,106 Routine vaccinations and prophylactic vaccinations are recommended against influenza A and B virus, pneumococci and Haemophilus influenzae in all patients with MM.107
As pomalidomide is structurally related to thalidomide, it is expected to have a teratogenic effect, and is contraindicated in pregnancy.103
Monitoring for patients on pomalidomide includes:
- Weekly complete blood counts, particularly in the first few cycles, to monitor neutropenia as well as thrombocytopenia
- Signs, symptoms and additive risk factors (smoking, hyperlipidaemia, hypertension) of VTE71
- International normalized ratio if the patient is on warfarin for thromboprophylaxis
- Pregnancy testing: obtain two negative pregnancy tests before starting lenalidomide – one 10–14 days prior to starting and the second 24 hours prior to starting. Pregnancy tests monthly thereafter in women with regular menstrual cycles, and every 2 weeks in women with irregular menstrual cycles103
Proteasome inhibitors
Ixazomib
Ixazomib was the first oral proteasome inhibitor (PI) to reach phase III clinical trials. Ixazomib in combination with lenalidomide and dexamethasone was approved in 2015 for the treatment of patients with relapsed/refractory MM.108 This all-oral triplet combination is the first of its kind to include both a PI and an IMiD, and it has shown excellent efficacy, safety and tolerability.39
Ixazomib is being tested as a single agent for maintenance therapy following ASCT or non-transplant induction, given its convenient, once-weekly, oral dosing and manageable toxicity profile.109–111 It is also being evaluated alone, or in combination, in patients with relapsed/refractory or newly diagnosed MM.111
Dose and schedule
In the approved combination of ixazomib, lenalidomide and dexamethasone, oral ixazomib is given 4 mg on days 1, 8 and 15 of a 28-day cycle.39,108
Side effects
Commonly observed toxicities are thrombocytopenia, nausea, vomiting, diarrhoea, constipation, rash, peripheral neuropathy, peripheral oedema and back pain, and they are mostly grade 1/2 in severity, manageable and reversible.112 Like with other PIs, herpes zoster rash is frequently seen with ixazomib use. The frequency decreases greatly with prophylaxis, which is recommended in all patients receiving ixazomib. Other side effects, including haematological events, occur much less frequently.112 The safety profile of ixazomib is summarized in Table 3.
Gastrointestinal effects: Gastrointestinal side effects are relatively frequent with ixazomib, with nausea, vomiting and diarrhoea seen in 29%, 23% and 45% respectively.112 These symptoms can be managed with supportive therapy, such as anti-diarrhoeal agents (primarily loperamide) and anti-emetics as needed, and dose adjustments.38,108
Skin rash: Rash has been reported at a frequency of 19%, typically occurring within 3 months of starting ixazomib, and is often self-limiting.108 Dose interruption and dose reduction can help with management. Additional medical management includes antihistamines (primarily cetirizine) or topical glucocorticoids.38
Peripheral neuropathy: In the single-agent trials of ixazomib, grade 3 peripheral neuropathy was reported in 2% of patients, and no grade 4 neuropathy was noted.108 Patients should be monitored for symptoms of peripheral neuropathy, and those experiencing new or worsening neuropathy may require dose modification or discontinuation.108
Hepatotoxicity: In trials of ixazomib combinations, elevations in serum aminotransferase levels were seen in approximately 10% of patients. However, values greater than five times the upper limit of normal were rare (<1%).108 Rarely, drug induced liver injury, hepatic steatosis, hepatitis cholestatic and hepatotoxicity have been reported, perhaps related to the production of a toxic intermediate.108
Cytopenias: Thrombocytopenia is seen commonly, especially in the middle of a 28-day cycle, but rapidly recovers to baseline by the time of initiation of the next cycle. Rarely (<1%), severe thrombocytopenia is seen.108 If, during treatment, platelets fall below 30,000/mm3, withhold treatment and adjust dosage. Rarely, severe neutropenia is seen with ixazomib use. If ANC falls to less than 500/mm3, withhold treatment and adjust dose.108
Monitoring for patients on ixazomib includes108
- Complete blood counts should be assessed monthly. More frequent monitoring during the first three cycles may be considered. Prior to initiating therapy, ANC should be >1,000/mm3.
- Monitor platelet counts at least monthly during treatment and adjust dosing as needed. Prior to initiating therapy, platelets should be >75,000/mm3.
- Gastrointestinal effects should be monitored and dosing adjusted for severe diarrhoea, constipation, nausea or vomiting.
- Hepatic enzymes should be monitored.108
Histone deacetylase inhibitors
Panobinostat
Panobinostat is an histone deacetylase inhibitor that, in combination with bortezomib and dexamethasone, was approved for treatment of patients with relapsed MM,113 but was been recently withdrawn as it was felt that there was a further need for adequate and controlled clinical research on the drug’s efficacy in this clinical setting.114
Dose and schedule
Panobinostat is given 20 mg three times weekly in a 21-day cycle (days 1, 3, 5, 8, 10 and 12) in patients with MM who have received at least two prior regimens.113
Side effects113
The main side effects of panobinostat are diarrhoea, peripheral neuropathy, asthenia and fatigue, and haematological side effects including neutropenia, thrombocytopenia and lymphocytopenia. Diarrhoea seen with panobinostat can be managed by anti-diarrhoeal medication, such as loperamide.
Cardiac AEs are rarely seen with this drug, but are worth noting. In randomized trials, asymptomatic cases of QT-segment prolongation, T-wave changes and ST–T segment changes were observed more frequently with panobinostat, compared with placebo.113 It should be used with caution in patients who have either pre-existing or high risk of corrected QT-segment (QTc) prolongation. If QTc increases to >480 ms, treatment should be interrupted and any electrolyte abnormalities corrected.113 It should not be initiated in patients with recent myocardial infarction or unstable angina. Other QTc prolonging medications should not be used concomitantly.
After dose interruptions, treatment is usually initiated at reduced doses. Doses are reduced in the increments of 5 mg, up until a minimum of 10 mg per dose three times a week. If the dose of panobinostat requires further reduction, it is discontinued altogether at this point.113
Monitoring for patients on panobinostat includes113
- Complete blood count should be monitored weekly, or more often if required. If low platelet counts are seen, hold therapy and restart with dose reductions once counts have recovered. Platelet transfusions are rarely needed;
- electrolytes, fluid intake and hydration status should be monitored if panobinostat-associated diarrhoea is severe.Electrocardiogram (ECG) should be done prior to starting therapy to verify that QTc is less than 450 ms. Repeat ECG and electrolytes periodically during treatment, as clinically indicated.
Selective inhibitors of nuclear transport
Selinexor
Selinexor is an orally bioavailable, highly potent, slowly reversible small molecule, classified as a selective inhibitor of nuclear transport. It is approved in combination with dexamethasone for patients with relapsed/refractory MM who have received at least four prior therapies and whose disease is refractory to at least two PIs, at least two IMiDs and an anti-CD38 monoclonal antibody.115
An all-oral combination of selinexor, pomalidomide and dexamethasone is under trial for patients with relapsed/refractory MM and newly diagnosed MM.116,117 The phase III BOSTON study (bortezomib, selinexor, and dexamethasone in patients with multiple myeloma (BOSTON); ClinicalTrials.gov identifier: NCT03110562) to evaluate the safety and efficacy of selinexor, bortezomib and low-dose dexamethasone in patients with relapsed/refractory MM is currently on-going.118
Dose and schedule
For the approved indication, selinexor is dosed at 80 mg/day twice weekly and combined with 20 mg dexamethasone twice weekly.113
Side effects
In clinical trials, the most common side effect of selinexor was nausea, which was seen in up to 68% of patients. Vomiting was seen in about one-third of patients.119,120 The rates of nausea and vomiting were the highest at the first dose, and decreased substantially with each cycle. About half of the patients experienced a decreased appetite and one-third lost weight. The intensity and duration of these symptoms can be mitigated by appropriate fluid and caloric intake, appetite stimulants and additional anti-nausea agents. In the selinexor trials, most patients studied received a mandatory 5-hydroxytryptamine (5-HT3) antagonist on the day of or after dosing to reduce nausea and vomiting. 119,120 Other agents used in these trials to improve nausea were: benzodiazepines as needed, neurokinin-1-receptor antagonists (rolapitant and aprepitant) and cannabinoid-receptor agonists (dronabinol).119,120 Prophylactic olanzapine and megestrol elicited some improvement as well.119,120
Thrombocytopenia is another frequently seen AE, with almost half of patients receiving selinexor affected.119 This may be partly related to selinexor inhibiting thrombopoietin signalling in early megakaryopoiesis.120 It can be managed with dose interruptions and thrombopoietin-receptor agonists if persistent.119,120
Another side effect of note is hyponatraemia, which can be seen in up to 25% of patients treated with selinexor, though it is generally asymptomatic and reversible with supportive care.119 The safety profile of selinexor is given in Table 3.
Monitoring for patients on selinexor includes:115
- Complete blood counts should be monitored weekly, and a 7–10-day drug holiday should be given on noting decreased platelet counts.
- Electrolyte levels should be monitored, particularly for sodium levels.
- Body weight should be monitored at baseline and during treatments, as clinically indicated.
B-cell lymphoma 2 inhibitors
Venetoclax
Venetoclax is a novel, orally bioavailable, small-molecule inhibitor that selectively targets B-cell lymphoma 2 (BCL2). BCL2 is a pro-survival protein, and its inhibition restores the apoptotic ability of malignant cells.121 Approximately 20% of patients with myeloma exhibit translocation t(11;14), which is associated with increased BCL2 expression, making venetoclax an attractive option in these patients.122 Venetoclax has been approved for many hematological malignancies, but has not yet been approved for MM. However,it has shown efficacy in patients with MM who have failed at least one prior treatment, particularly those with a t(11;14) translocation.122,123
Dosing and schedule
Venetoclax is administered in doses ranging from 300 to 1,200 mg daily in a 21-day cycle. If combined with dexamethasone, dexamethasone is given 40 mg weekly.24–26
Side effects
Cytopenias are the most common side effect of venetoclax. Nausea and diarrhoea are occasionally seen, but are self-limiting. Of note, hypophosphataemia has been seen with venetoclax dosing.26,123 The safety profile of venetoclax is summarized in Table 3.
Administering live vaccines to patients on venetoclax is cautioned against, as the safety, efficacy and after effects of live-attenuated vaccines in venetoclax therapy have not been studied yet.26,124 It is advised to wait until B-cell recovery for administration of these vaccines.123
Foetal toxicity
Animal studies have shown post-implantation loss and decreased foetal weight, and adequate data of the safety profile of venetoclax does not exist in pregnant females as of yet. Hence, pregnancy tests should be performed for all women of childbearing potential prior to starting venetoclax, and contraception should be used throughout treatment, and for at least 30 days after completing therapy.124
Monitoring for patients on venetoclax includes:123
- Complete blood counts should be monitored and managed appropriately;
- patients should be monitored for signs of infection, which should be managed as medically appropriate. Hold drug if grade ≥3 infection occurs.123
Alkylating agents
Melphalan
Melphalan + prednisone is a well-studied oral regimen and was historically the standard of care for over 40 years in patients with newly diagnosed myeloma.125 Adding thalidomide as a third agent improves efficacy, but increases toxicity. The use of melphalan has declined due to its toxicity and the availability of more effective regimens. Currently, oral melphalan is approved as a palliative therapy in patients with MM.76 Of note, high dose melphalan has a higher degree of bone marrow suppression and subsequently a higher recovery time, therefore it is used in combination with ASCT, and has become the standard of care in patients with newly diagnosed disease, eligible for haematopoietic stem cell transplantation in patients aged <65 years. As many patients with MM are elderly, with a median age of diagnosis ~70 years, a significant proportion of patients with MM cannot receive high-dose melphalan due to bone marrow suppression and toxicity and with it, increased mortality.126
Dose and schedule76
Oral melphalan is currently approved for patients with MM at a dosage of 6 mg daily (3 tablets, which may be given together). Trials have demonstrated efficacy of doses ranging from 4 to 8 mg/m2 as listed below. The dose is adjusted based on blood counts, which are checked weekly. It is usually combined with prednisone at 40–60 mg/m2.
Side effects
Oral melphalan has a unique AE profile, and should not be administered in patients with prior resistance or hypersensitivity to this chemotherapeutic agent. Some commonly seen ADRs include mucositis and bone marrow suppression. Rarely, secondary malignancies and pulmonary toxicity have also been seen.76 The safety profile of melphalan is summarized in Table 3.
Mucositis: Mucositis and injury to the gastrointestinal tract are the most common acute AEs seen with melphalan. Mucositis was seen as frequently as in 75% of patients on melphalan in one study, with severe mucositis seen in 21%. Severity of mucositis was dose dependent.74 Oropharyngeal mucositis is usually painful and sometimes involves the supraglottic area, which may necessitate intubation. Intestinal injury results in nausea, vomiting, diarrhoea, cramping and occasionally, acute abdomen. Recipients often require narcotics and parenteral alimentation.74,127,128
To reduce the likelihood of mucositis, patients are advised to eat ice chips to restrict blood flow to the oral mucosa and to reduce drug delivery to the area (referred to as cryotherapy). Palifermin – a keratinocyte growth factor – seems to have a protective effect. Amifostine – a cytoprotectant – scavenges free radicals to facilitate safer use of high-dose melphalan.127,128
Bone marrow suppression: Bone marrow suppression frequently occurs, but is reversible if melphalan is withdrawn early on. Irreversible cases of bone marrow failure due to melphalan use have been reported.76 Melphalan damages the haematopoietic stem-cell compartment, as well as increases the risk of myelodysplasia following transplantation. Patients eligible for haematopoietic stem cell transplantation should not receive melphalan, even orally. They should undergo mobilization and harvesting of peripheral blood progenitor cells before receiving melphalan in the setting of limited treatment options.129,130 It was also noted that melphalan’s haematological toxicity was significantly linked to renal dysfunction. Melphalan, which is a small-cell toxin, may compromise stem-cell reserve and should be avoided in patients that are candidates for stem-cell transplantation.131
Pulmonary toxicity: Pulmonary toxicity is rare with melphalan treatment, but cases of diffuse interstitial lung fibrosis pathologically resembling busulfan-induced lung injury have been reported following melphalan therapy in patients with MM.132,133 Other pulmonary complications include acute bronchoconstriction, diffuse interstitial pneumonitis and fibrosis.134
Fertility impairment: Of note, melphalan can impair fertility. Melphalan suppresses ovarian function in premenopausal women, resulting in amenorrhoea for many.76 Reversible and irreversible testicular suppression has also been reported.76 Haemorrhagic cystitis is also rarely seen with melphalan.66
Monitoring for patients on melphalan includes:76
- Complete blood count should be performed at the start of therapy and before each subsequent dose, and further therapy should be withheld until counts have sufficiently recovered (leukocytes <3,000/μL and platelets <100,000/μL). Clinicians should consider adjusting dose based on blood counts at their lowest point and on the day of treatment.76
- Observe patients for signs of bone marrow suppression (e.g. severe infections, bleeding and symptomatic anaemia).
Cyclophosphamide
A combination of oral cyclophosphamide and steroids (prednisone or dexamethasone) were historically used in patients with advanced MM as an alternative to melphalan-based regimens. Cyclophosphamide did not need dose adjustments for renal insufficiency, making it an attractive option at the time, with lesser bone marrow suppression seen as well;135 though it has fallen out of favour as newer drugs have arrived with fewer side effects and easier administration.136 The efficacy of cyclophosphamide is summarized in Table 1.
Dose and schedule
Cyclophosphamide is administered at the dose of 100–500 mg, and can be given daily to once weekly. It is given concurrently with prednisone, which can be dosed ranging from 10 mg to 100 mg.
Side effects137
Vomiting and nausea are frequent within the first 12 hours of cyclophosphamide administration. Persistent and intractable nausea and vomiting are managed with antiemetics, such as ondansetron, other 5-HT3 receptor antagonists, aprepitant, dexamethasone and others.138,139 Also, myelosuppression is seen in a significant number of patients, but is reversible and improves with dose reduction or interruption.32 It has also been reported to cause foetal harm in some cases, and hence women of childbearing ages are advised to avoid conception while on cyclophosphamide.138 Also, secondary primary malignancies can develop many years after discontinuation of cyclophosphamide therapy.137
Hemorrhagic cystitis: Chronic or recurrent haemorrhagic cystitis is of particular concern with cyclophosphamide treatment. It arises when cyclophosphamide is administered without adequate hydration or without concurrent mesna administration.32 Haemorrhagic cystitis is a complex inflammatory response that occurs due to the production of the urotoxic hepatic metabolite, acrolein. Acrolein activates immunocompetent cells and the production of many pro-inflammatory agents, resulting in ulceration, swelling and bleeding of bladder mucosa. Mesna conjugates acrolein in urine, and is therefore used to prevent severe bladder toxicity.137 In addition to mesna, patients on oral cyclophosphamide should increase their fluid intake, with forced diuresis and frequent bladder emptying.140
Gonadal damage: Cyclophosphamide is gonadotoxic. It destroys ovarian cells by cross-linking DNA, and specifically depletes primordial follicles by cross-linking DNA and stimulating apoptosis pathways. Several studies suggest that adjuvant therapies to protect primordial germ cells from these off-target effects may reduce this risk.141,142 In men, cyclophosphamide produces adducts and cross-links DNA, leading to long-term azoospermia. The effect is dose dependent, with patients on lower doses recovering to normospermic levels within 1–3 years, and those on higher doses developing more prolonged, even permanent effects.143
Cardiopulmonary toxicities: Although rare, cardiopulmonary toxicity has been documented following treatment with cyclophosphamide in the form of tachyarrhythmias, hypotension, heart failure, myocarditis, pericardial diseases, pneumonitis and respiratory failure. Cardiotoxicity is dose dependent, and evident as reversible changes in ECG and ventricular mass. Twice-daily regimens of cyclophosphamide have much less of an impact on systolic function than high-dose, once-daily regimens. Severe cases related to high doses of cyclophosphamide may present as haemorrhagic myocarditis, cardiac tamponade and cardiogenic shock. The minimum doses for avoiding cardiotoxicity altogether are unknown, but it is worth mentioning that, thus far, no reports on cyclophosphamide-related cardiotoxicity exist for doses under 100 mg/kg.58,144
Monitoring for patients on cyclophosphamide includes:137
- Complete blood count monitoring is essential. Dose interruptions and discontinuation is needed if neutrophils drop below 1,000/mm3 or platelets below 50,000/mm3, or if a patient develops a serious infection. The lowest leukocyte and thrombocyte counts are usually reached in the first 2 weeks of treatment.
- Before starting treatment, exclude or correct any urinary tract obstructions. Routinely test urine for erythrocytes and other signs of uro- or nephrotoxicity. Monitor for urinary signs and symptoms like gross haematuria, burning, dysuria, urgency and incontinence.
All-oral combination regimens
For myeloma, drugs are given in combinations, either in pairs, doublets or triplets based on risk stratification, genetics, disease stage, transplant eligibility and progression of disease.145 Many all-oral combinations significantly improve outcomes and survival compared with conventional therapies. They pose a very attractive option given their ease of administration, the convenience of outpatient dosing and their decreased need for hospital visits. The phase III trials of all such combinations, along with their adverse drug reactions of interest, are outlined in Table 2.
Discussion
Conventionally, myeloma management has included drug combinations that include oral and intravenous drugs. The current treatment paradigm in myeloma increasingly relies on continuous therapy until disease progression, which in the context of intravenous therapies, calls for frequent clinic visits. The cost associated with treating and managing myeloma is among the highest for all malignancies, and aside from the actual therapies, results from in-hospital infusions, frequent clinic visits and travel to receive these comprehensive regimens.146,147 Furthermore, all-oral combination therapies have shown good outcomes, as outlined in Table 2. Shifting towards an all-oral regimen will reduce financial burden, mitigate a large proportion of time spent travelling and in hospital, and prevent excess exposure of these immunocompromised individuals to nosocomial organisms.1 With the outbreak of a global pandemic, as well as the advent of oral therapies, there has been a paradigm shift towards preventing unnecessary hospital and clinic visits. As such, healthcare providers, as well as caregivers, may benefit from a decreased administrative burden. Lastly, a thorough discussion and focus on patient preference, comorbidities, availability of a caregiver, functional status and ability to report adverse effects is imperative in choosing the final line of therapy, and this decision must be individualized for each patient.