Gastro-oesophageal cancers (GEC), which include both gastric (GC) and oesophageal cancers (EC), are among the most common cancers globally.1 In the USA, the American Cancer Society estimated 20,640 new diagnoses of EC and 26,380 new diagnoses of GC, each contributing to 16,410 and 11,090 deaths, respectively, in 2022 alone.2,3 GEC is divided based on the histology of squamous cell carcinoma (SCC) and adenocarcinoma.4 Worldwide, SCC is considered the most common histological subtype; however, adenocarcinoma has become the most common subtype in the USA and developed countries in recent decades.5 This notable prevalence of adenocarcinoma in developed countries is attributed to the increasing rate of obesity, gastroesophageal reflux disease and the decreased rate of smoking and alcohol consumption, which commonly contribute to SCC.6
Helicobacter pylori infection, obesity, smoking, consumption of red meat and alcohol, and low socioeconomic status are all key risk factors for GC.7 Consumption of alcoholic beverages and tobacco use are two major risk factors also associated with EC.8 Specifically, SCC of the oesophagus has also been linked to certain carcinogens, such as the nitrosamines present in salted vegetables and preserved salmon.9 The pathophysiology appears to be associated with squamous epithelial inflammation, which progresses to dysplasia and in situ malignancy.10
Despite recent advancements in diagnostics, most patients with GEC present with incurable diseases, with symptoms including weight loss, abdominal pain, dysphagia, nausea and vomiting.11,12 In patients who present with localized disease, surgery is the only potentially curative treatment, while neoadjuvant and adjuvant therapies should be integrated with surgery in locally advanced diseases. Even with the progress made in the recent decade by integrating neoadjuvant and adjuvant therapies in locally advanced disease, the survival rate of patients with GEC remains low.13
Our insights into the molecular pathogenesis and physiology of cancer have vastly improved over the last decade. In 2014, the Cancer Genome Atlas Research Network defined the following genomic subtypes of GC: Epstein–Barr virus-infected tumours, microsatellite instable (MSI) tumours, gnomically stable tumours and chromosomally unstable tumours.14 These findings helped us to understand the signalling pathways in GEC and paved the way for developing targeted therapies.
However, there have been relatively few approved target-directed methods for treating metastatic GEC to date. Trastuzumab, an anti-human epidermal growth factor receptor-2 (HER2) drug, was the first targeted therapy approved for HER2 high-expressing advanced GEC.15,16 Subsequently, ramucirumab, an antiangiogenic agent, was approved for the second-line setting as a monotherapy or in combination with paclitaxel.17–19 In recent years, anti-programmed death receptor 1 (PD-1) agents such as nivolumab and pembrolizumab have been approved in the USA in combination with chemotherapy or as monotherapy in a different treatment setting for advanced GEC.1 Despite the US Food and Drug Administration (FDA) approval of several of these therapies for GEC, the disease continues to have an overall poor prognosis (Figure 1). In this article, we will review the evolution of approved targeted drugs, as well as the current state of targeted treatments and immunotherapies in GEC.
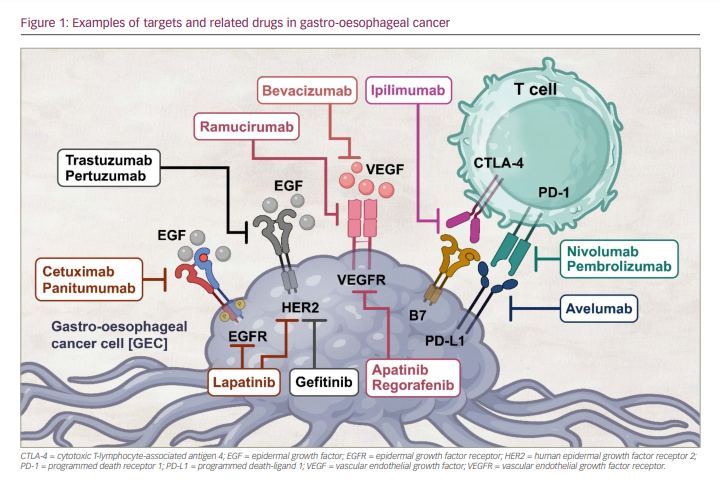
Targeted therapy
Targeting the human epidermal growth factor receptor 2
HER2, a member of the HER/epidermal growth factor receptor (EGFR)/ErbB family, is a receptor tyrosine kinase that sequesters to the cell membrane. Moreover, it further regulates cellular proliferation and differentiation and cancer progression extracellular–intracellular communication.8 Given the success of drugs and agents targeting HER2 in treating breast cancer, HER2 has also become an appropriate target for patients with metastatic GC or gastro-oesophageal junction cancer (GEJC).20–22
Trastuzumab, the first FDA-approved anti-HER2 directed therapy, inhibits HER2 by binding to the juxtamembrane portion of the HER2 extracellular domain. Consequently, it prevents the activation of intracellular kinase in HER2 and enables the cyclin-dependent kinase (CDK) inhibitor to be redirected from p27 to CDK2, allowing the HER2 overexpressing cells to stop proliferation.9,23 In preclinical experiments, trastuzumab enhances antibody-dependent cell toxicity, and trastuzumab-bound HER2 receptors mobilize cytotoxic T cells to destroy the cell.14,23
The ToGA study (A randomized, open-label study of the effect of first-line trastuzumab in combination with a fluoropyrimidine and cisplatin versus chemotherapy alone on overall survival in patients with HER2-positive advanced gastric cancer; ClinicalTrials.gov identifier: NCT01041404), a randomized phase III trial, compared fluorouracil and cisplatin (chemotherapy) to fluorouracil and cisplatin combined with trastuzumab in patients with HER2-positive (3+ on immunohistochemistry or HER2:CEP17 ratio ≥2 on fluorescence in situ hybridization [FISH]) and advanced GC or GEJC.15,24 In the trastuzumab arm, there was an improvement in overall survival (OS) compared with chemotherapy alone (13.8 versus 11.1 months for trastuzumab plus chemotherapy versus chemotherapy alone; p=0.0046) and progression-free survival (PFS) (6.7 versus 5.5 months for trastuzumab plus chemotherapy versus chemotherapy alone; p=0.0002).14 Notably, the most benefit was seen in patients with FISH positive/immunohistochemistry (IHC) 2+ or IHC 3+ HER2-positive tumours. As a result of the ToGA trial, the FDA approved trastuzumab for use as a first-line treatment in combination with chemotherapy in patients with metastatic HER2-positive metastatic gastric or gastro-oesophageal junction (GEJ) adenocarcinoma.25
The benefit of continuing trastuzumab following GC and GEJC progression was investigated.26 In the phase II T-ACT study (Randomized phase II study comparing trastuzumab plus weekly paclitaxel with weekly paclitaxel alone for patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum; University Hospital Medical Information Network identifier: UMIN000009297), patients were assigned to paclitaxel with or without trastuzumab.26,27 The study revealed no benefit from trastuzumab beyond progression, which was ascribed to a reduction of HER2 expression in individuals who had previously had trastuzumab therapy.
After trastuzumab, the next HER2-directed therapy to be investigated was trastuzumab deruxtecan (T-DXd). T-DXd is a HER2-targeting antibody–drug conjugate of the humanized anti-HER2 antibody covalently linked to deruxtecan, which is a topoisomerase I inhibitor.28 This conjugate allows for targeted delivery of the cytotoxic deruxtecan, where the drug exerts its effect by inhibiting topoisomerase I, resulting in DNA replication inhibition and cell cycle arrest, ultimately causing tumour cell apoptosis.15 While most HER2-targeted therapy failed to show any benefit after disease progression on initial trastuzumab-based therapy, T-DXd showed promise. As part of the DESTINY-Gastric01 trial (A phase II, multicenter, open-label study of DS-8201a in subjects with HER2-expressing advanced gastric or gastroesophageal junction adenocarcinoma; ClinicalTrials.gov identifier: NCT03329690), T-DXd was compared with chemotherapy (irinotecan or paclitaxel) in 187 patients with HER2-positive GC or GEJC who had received two or more prior therapies, including trastuzumab.28,29 The primary outcome, objective response rate (ORR), was significantly better in the T-DXd group when compared with chemotherapy (51% versus 14%; p=0.001). As a crucial secondary endpoint, T-DXd demonstrated superior OS compared with chemotherapy (median OS: 12.5 versus 8.4 months; hazard ratio [HR]: 0.59; p=0.01) and PFS (5.6 versus 3.5 months; HR: 0.47). Based on this trial, FDA granted T-DXd approval for patients with HER2-positive unresectable advanced or recurrent GC who had disease progression on a prior trastuzumab-based regimen.30
Another HER2-targeted therapy is ado-trastuzumab emtansine (T-DM1), which binds to HER2 and enables the HER2–T-DM1 complex to enter cells via receptor-mediated endocytosis.23 As the linker is designed to be stable in both the systemic circulation and tumour microenvironment, the active T-DM1 is released exclusively intracellularly by proteolytic degradation of the antibody portion of T-DM1 in the lysosome.31 DM1-containing metabolites block microtubule formation after being released from the lysosome, eventually leading to cell death.32
Trastuzumab emtansine was extensively studied in an open-label, adaptive phase II/III study (A randomized, multicenter, adaptive phase II/III study to evaluate the efficacy and safety of trastuzumab emtansine [T-DM1] versus taxane [docetaxel or paclitaxel] in patients with previously treated locally advanced or metastatic HER2-positive gastric cancer, including adenocarcinoma of the gastroesophageal junction; ClinicalTrials.gov identifier: NCT01641939) to assess its efficacy and tolerability in patients who had previously undergone treatment for HER2-positive advanced GC.33,34 At a dose of 24 mg/kg weekly, trastuzumab emtansine had a similar median OS to taxane (7.9 versus 8.6 months; HR: 1.15; 95% confidence interval [CI]: 0.87–1.15; p=0.86). Hence, trastuzumab emtansine was not found to be superior to taxanes in previously treated patients with HER2-positive advanced GC.33
Lapatinib is a dual tyrosine kinase inhibitor that targets and inhibits HER2 and EGFR.31 It suppresses the activation of the three primary EGFR and HER2 downstream signalling pathways: MAPK, PI3K-AKT and PLC.33 This process also increases the expression of p38, a component that is directly involved in apoptosis.35 In the TRIO-013/LOGiC trial (A phase III study for ErbB2 positive advanced or metastatic gastric, oesophageal, or gastroesophageal junction adenocarcinoma treated with capecitabine plus oxaliplatin with or without lapatinib; ClinicalTrials.gov identifier: NCT00680901), 545 treatment-naïve patients with advanced HER2-positive gastric, oesophageal and GEJ adenocarcinoma were randomly assigned to receive chemotherapy (capecitabine and oxaliplatin) with or without lapatinib. The inclusion of lapatinib did not produce a statistically significant improvement compared with chemotherapy alone (median OS: 12.2 versus 10.5 months; HR: 0.91; p=0.349).36,37 In the second-line setting, the TyTAN study (A randomized, multicentre, open-label, phase III study of lapatinib [GW572016] in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of ErbB2 amplified advanced gastric cancer; ClinicalTrials.gov identifier: NCT00486954) evaluated lapatinib’s survival advantage when combined with paclitaxel (chemotherapy) in HER2-amplified advanced gastric adenocarcinoma irrespective of HER2 expression.35,38 Patients treated with lapatinib plus paclitaxel had a median OS of 11.0 months compared with 8.9 months in patients treated with paclitaxel alone; furthermore, the difference between the two groups was not statistically significant (p=0.1044).35 Therefore, no FDA approval was granted for lapatinib in patients with GC.
The most recent advance in treating HER2-positive advanced gastric or oesophagogastric junction (EGJ) adenocarcinoma was the combination of pembrolizumab and trastuzumab in the phase III KEYNOTE-811 trial (A phase III, randomized, double-blind trial comparing trastuzumab plus chemotherapy and pembrolizumab with trastuzumab plus chemotherapy and placebo as first-line treatment in participants with HER2 positive advanced gastric or gastroesophageal junction adenocarcinoma; ClinicalTrials.gov identifier: NCT03615326).39,40 The study enrolled 692 patients who had not previously received systemic therapy; the patients were randomized 1:1 to either pembrolizumab or placebo given with trastuzumab plus three-week cycles of platinum-containing cytotoxic chemotherapy (cisplatin plus fluorouracil or oxiplatin and capecitabine).
Presented at the 2021 American Society of Clinical Oncology annual meeting, the interim results of the KEYNOTE-811 trial showed that patients who had received pembrolizumab had a higher ORR than those who had received placebo (74.4% versus 51.9%). Further, their median duration of response was 10.6 months, compared with 9.5 months for the placebo group.41 Pembrolizumab also provided a higher complete response rate than placebo (3.1% versus 11.3%). In addition, pembrolizumab produced more durable responses at 6 months than placebo (70.3% versus 61.4%).41
In light of the interim results mentioned above, the FDA approved pembrolizumab used with trastuzumab, fluoropyrimidine and platinum-based chemotherapy as the first-line treatment for advanced HER2-positive advanced gastric or EGJ adenocarcinoma. The clinical trials of drugs targeting HER2 are presented in Table 1.15,26–28,33,35,36,39,42–47
Targeting angiogenesis
Tumours and stromal cells release vascular endothelial growth factors (VEGFs), which work in autocrine or paracrine pathways by interacting with VEGF receptors (VEGFRs), including VEGFR1, VEGFR2 and VEGFR3.39 A VEGF/VEGFR interaction activates a number of signalling cascades, including extracellularly regulated protein kinases 1/2 (ERK1/2) and phosphatidylinositol 3-kinase/protein kinase B/Akt (PI3K/AKT), resulting in angiogenesis with enhanced cell proliferation, recruitment and survival.41 For these reasons, the VEGF/VEGFR signalling pathway is naturally of interest in GC and EC therapy since VEGF overexpression is significantly associated with poor prognosis.26,42
Ramucirumab is a novel humanized immunoglobulin G1 monoclonal antibody that inhibits VEGFR2 to block angiogenesis and trigger tumour cell death.28 It was tested in the RAINBOW phase III trial (A randomized, multicenter, double-blind, placebo-controlled phase 3 study of weekly paclitaxel with or without ramucirumab [IMC-1121B] drug product in patients with metastatic gastric adenocarcinoma, refractory to or progressive after first-line therapy with platinum and fluoropyrimidine; ClinicalTrials.gov identifier: NCT01170663) for patients with advanced gastric or GEJ adenocarcinomas who had previously been treated with platinum- and fluoropyrimidine-containing regimens (chemotherapy).17,48 Patients were randomized to paclitaxel with ramucirumab or with placebo. The study showed that the addition of ramucirumab to paclitaxel compared with chemotherapy alone prolonged median OS (9.6 versus 7.4 months; p=0.017) and PFS (4.4 versus 2.9 months; p<0.001). The most common adverse events noted for patients in the ramucirumab group compared with those in the placebo group were grade 3 or 4 neutropenia (41% versus 19%) and grade ≥3 hypertension (14% versus 2%).
The phase III REGARD trial (A phase III, randomized, double-blinded study of IMC-1121B and best supportive care [BSC] versus placebo and BSC in the treatment of metastatic gastric or gastroesophageal junction adenocarcinoma following disease progression on first-line platinum- or fluoropyrimidine-containing combination therapy; ClinicalTrials.gov identifier: NCT00917384) investigated ramucirumab monotherapy for advanced gastric or GEJ adenocarcinoma that had progressed on first-line platinum- or fluoropyrimidine-based therapy.49,50 Ramucirumab monotherapy with best supportive care (BSC) was compared with placebo with BSC.49 Patients in the ramucirumab group had a median OS of 5.2 months, while those in the placebo group had a median OS of 3.8 months (HR: 0.776; 95% CI: 0.603–0.998; p=0.047). After adjusting for additional predictive factors (tumour site, functional status and presence of peritoneal metastases) in the multivariate analysis, ramucirumab’s survival benefit remained (p=0.042). Hypertension was more common in the ramucirumab group than in the placebo group (16% versus 8%), but other adverse events were generally comparable between the two groups.
Bevacizumab is a VEGF-A targeted monoclonal antibody. Blocking VEGF-A from engaging with VEGFR2 can improve vascular permeability and further limit tumour growth.49 The AVAGAST trial (A double-blind, randomized, multicenter, phase III study of bevacizumab in combination with capecitabine and cisplatin versus placebo in combination with capecitabine and cisplatin, as first-line therapy in patients with advanced gastric cancer; ClinicalTrials.gov identifier: NCT00548548) was the first basket trial to assess the efficacy and safety of bevacizumab with chemotherapy in patients, compared with chemotherapy alone presented with an inoperable solid tumour.51,52 Chemotherapy plus bevacizumab trended towards improved survival compared with chemotherapy plus placebo (median OS: 12.1 versus 10.1 months; HR: 0.87; 95% CI: 0.73–1.03; p=0.1002). Despite a notable improvement in the bevacizumab group compared with the placebo group in PFS (6.7 versus 5.3 months; HR: 0.80; 95% CI: 0.68–0.93; p=0.0037) and ORR (46.0% versus 37.4%; p=0.0315), the study failed to meet the primary OS goal; therefore, bevacizumab is not preferentially used to treat GC.
The clinical trials targeting angiogenesis are included in Table 2.17,49–60
Targeting epidermal growth factor receptor
The EGFR is a tyrosine kinase that belongs to the ErbB family of receptors. When it binds to its ligand epidermal growth factor (EGF), it undergoes homodimerization or heterodimerization, followed by autophosphorylation. As a result of the whole process, downstream effectors, such as the RAS-RAF-MEK-ERK-MAPK and PI3K-AKT-mTOR pathways, are activated and perform a vital role in cellular proliferation, differentiation and survival.60,61 Therefore, aberrant EGFR activation, such as receptor overexpression and the heterodimerization of ligand-dependent receptors, is linked to the development of cancer.
While many EGFR inhibitors are now available, none have shown promising results in treating GECs. Currently, more attention is being turned towards innovative approaches for delivering EGFR-targeted therapy. Various drug delivery systems have been developed, including liposomes, nanobullets, exomes and macrovesicles.62 Such technologies show promise for more efficient drug delivery into the tumour microenvironments and intracellularly.62
To explore the EGFR inhibitors studied in GEC, we will start with cetuximab, a monoclonal antibody that has demonstrated activity in other gastrointestinal malignancies, such as colon adenocarcinoma.63 In the phase III EXPAND trial (Open-label, randomized, controlled, multicenter phase III study investigating cetuximab in combination with capecitabine [Xeloda, X] and cisplatin [P] versus XP alone as first-line treatment for subjects with advanced gastric adenocarcinoma, including adenocarcinoma of the gastroesophageal junction; ClinicalTrials.gov identifier: NCT00678535), 904 treatment-naïve participants with metastatic gastric and GEJ adenocarcinoma were treated with cisplatin and capecitabine (chemotherapy) with or without cetuximab.64,65 The study found that, compared with chemotherapy alone, the addition of cetuximab demonstrated an inferior median PFS (4.4 versus 5.6 months; HR: 1.09; 95% CI: 0.92–1.29; p=0.32) and OS (9.4 versus 10.7 months; HR: 1.00; 95% CI: 0.87–1.12; p=0.95).
A phase II study of cetuximab monotherapy in patients with metastatic EC who had previously failed first-line chemotherapy was conducted, with the primary endpoint of OS (Cetuximab as second-line therapy in patients with metastatic esophageal cancer – Phase II; ClinicalTrials.gov identifier: NCT00096031).66,67 The median OS was 4.0 months (95% CI: 3.2–5.9), and the median PFS was 1.8 months (95% CI: 1.7–1.9). Overall, the study failed to meet the primary survival endpoint.
Panitumumab is another human monoclonal antibody against EGFR for the treatment of colorectal cancer that was also tested for EC.68 In the AIO/EORTC trial (An open-label, randomized phase III trial of cisplatin and 5-fluorouracil with or without panitumumab for patients with nonresectable, advanced or metastatic esophageal squamous cell cancer; ClinicalTrials.gov identifier; NCT01627379), patients with metastatic oesophageal SCC were randomized to cisplatin and fluorouracil (chemotherapy) with or without panitumumab. However, the study failed to show any improvement in ORR, PFS or OS.69,70
Targeting neurotrophic tropomyosin-receptor
kinase fusion
Neurotrophic tropomyosin-receptor kinase (NTRK) gene rearrangements have been reported in a broad spectrum of tumours, including thyroid, head and neck, lung, and colon cancers. NTRK fusion plays a major role in oncogenesis by activating TRK receptors and subsequently activating the downstream pathways. Larotrectinib and entrectinib were approved by the FDA in 2018 and 2019, respectively, for all solid tumours with NTRK gene fusion for therapy in the subsequent-line setting.71,72 While the data about NTRK inhibitors in GEC are limited to case reports, the tyrosine kinase inhibitor is a novel class of drugs with a potential for good response.73,74
Immunotherapy
The role of immunotherapy in patients with advanced unresectable GC, GEC and EC was first studied in 2017 under a series of KEYNOTE trials, which led to the expedited FDA approval of pembrolizumab to treat patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumours, including gastro-oesophageal malignancies.75 The activity of pembrolizumab has been seen in the KEYNOTE-158 trial (A clinical trial of pembrolizumab [MK-3475] evaluating predictive biomarkers in subjects with advanced solid tumours; ClinicalTrials.gov identifier NCT02628067), where 24 patients (10.3%) with gastric malignancies out of the 233 patients with non-colorectal MSI-H/dMMR cancers were included.76 In this subset of patients with GEC, pembrolizumab showed an ORR of 45.8%, a median PFS of 4.1 months and a median OS of 23.5 months.
The benefit of pembrolizumab monotherapy was not limited to MSI-H tumours. In the KEYNOTE-181 trial (A phase III randomized open-label study of single-agent pembrolizumab versus physicians’ choice of single-agent docetaxel, paclitaxel, or irinotecan in subjects with advanced/metastatic adenocarcinoma and squamous cell carcinoma of the esophagus that have progressed after first-line standard therapy [KEYNOTE-181]; ClinicalTrials.gov identifier: NCT02564263), patients with advanced SCC of the oesophagus and GEJ adenocarcinoma (regardless of MSI status) who had progressed after first-line treatment were randomized to receive pembrolizumab or chemotherapy.77,78 While patients with adenocarcinoma did not experience survival benefit, the sub-analysis for patients with SCC histology showed a survival advantage of pembrolizumab over chemotherapy if the programmed death-ligand 1 (PD-L1) combined positive score (CPS) score was >10 (HR: 0.64; 95% CI: 0.46–0.90). The above finding led to the FDA approval of pembrolizumab monotherapy in the second-line setting for patients with SCC who had PD-L1 CPS ≥10.79
The KEYNOTE-061 trial (A phase III, randomized, open-label clinical trial of pembrolizumab [MK-3475] versus paclitaxel in subjects with advanced gastric or gastroesophageal junction adenocarcinoma who progressed after first-line therapy with platinum and fluoropyrimidine; ClinicalTrials.gov identifier: NCT02370498) compared the efficacy of pembrolizumab monotherapy versus paclitaxel alone in patients with advanced gastric and GEJ adenocarcinomas with PD-L1 CPS ≥1.80,81 The study failed to show any statistical survival benefit of pembrolizumab over chemotherapy (median OS: 9.1 versus 8.3 months; HR: 0.82; 95% CI: 0.66–1.03). However, updated results of the study later showed the survival benefit of pembrolizumab in patients with CPS >5 (median OS: 10.4 versus 8.3 months; HR: 0.72; 95% CI: 0.53–0.99) and >10 (median OS: 10.4 versus 8.0 months; HR: 0.69; 95% CI: 0.46–1.05).82
A number of studies have tested pembrolizumab in combination with chemotherapy. In the phase III KEYNOTE-062 trial (A randomized, active-controlled, partially blinded, biomarker select, phase III clinical trial of pembrolizumab as monotherapy and in combination with cisplatin + 5-fluorouracil versus placebo + cisplatin + 5-fluorouracil as first-line treatment in subjects with advanced gastric or gastroesophageal junction [GEJ] adenocarcinoma; ClinicalTrials.gov identifier: NCT02494583), patients with locally advanced or metastatic GC and GEJ cancer with a PD-L1 CPS ≥1 were randomly assigned to receive pembrolizumab alone, chemotherapy plus pembrolizumab or chemotherapy plus placebo.83,84 Pembrolizumab monotherapy was non-inferior to chemotherapy for OS in patients with CPS ≥1 (median OS: 10.6 versus 11.1 months; HR: 0.91; 99.2% CI: 0.69–1.18). The subset of patients with CPS >10 demonstrated a prolonged median OS with pembrolizumab monotherapy compared with chemotherapy alone (17.4 versus 10.8 months; HR: 0.69; 95% CI: 0.49–0.97); however, the statistical significance was not tested. In the subset of patients with MSI-H tumours and PD-L1 CPS >10, median OS was prolonged (median, not reached; HR: 0.21; 95% CI: 0.06–0.83).
In the phase III KEYNOTE-590 trial (A randomized, double-blind, placebo-controlled phase III clinical trial of pembrolizumab [MK-3475] in combination with cisplatin and 5-fluorouracil versus placebo in combination with cisplatin and 5-fluorouracil as first-line treatment in subjects with advanced/metastatic esophageal carcinoma [KEYNOTE-590]; ClinicalTrials.gov identifier: NCT03189719), patients with metastatic EC or GEJC were assigned to either pembrolizumab or placebo, as well as 5-fluorouracil and cisplatin (chemotherapy), irrespective of PD-L1 status.85,86 The study showed the superiority of pembrolizumab plus chemotherapy compared with chemotherapy alone in all patients with oesophageal SCC, regardless of histology or PD-L1 CPS (median OS: 12.4 versus 9.8 months; HR: 0.73; 95% CI: 0·62–0·86; p<0. 0001). The survival benefit added by pembrolizumab compared with placebo was seen more notably in patients with SCC histology, who reported a median OS of 13.9 versus 8.8 months compared with patients with adenocarcinoma, who reported a median OS of 11.6 versus 9.9 months. Furthermore, OS was improved with the addition of pembrolizumab in patients with tumours with CPS ≥10 (13.5 versus 9.4 months), compared with CPS <10 (10.5 versus 10.6 months).
Although the previously described KEYNOTE-062 trial did not show the superiority of pembrolizumab plus chemotherapy over chemotherapy alone in patients with untreated advanced GC and GEJC, the CheckMate 649 trial (A randomized, multicenter, open-label, phase III study of nivolumab plus ipilimumab or nivolumab in combination with oxaliplatin plus fluoropyrimidine versus oxaliplatin plus fluoropyrimidine in subjects with previously untreated advanced or metastatic gastric or gastroesophageal junction cancer; ClinicalTrials.gov identifier: NCT02872116) showed benefit in adding anti-PD-1 nivolumab to chemotherapy.83,87,88 The combination of nivolumab and chemotherapy was assessed in this phase III trial, where 1,581 patients with previously untreated HER2-negative advanced or metastatic gastric, EGJ or oesophageal adenocarcinoma (regardless of PD-L1 expression) were randomly assigned to nivolumab plus chemotherapy (oxiplatin and capecitabine, or folinic acid, fluorouracil and oxiplatin) or chemotherapy alone.87
In patients with a PD-L1 CPS ≥5 (60% of patients), the combined chemotherapy and immunotherapy group had a 29% reduction in the risk of death compared with the chemotherapy alone group (HR: 0.71; 98.4% CI: 0.59–0.86; p<0.0001), as well as a 3.3-month improvement in median OS (14.4 versus 11.1 months; p<0.0001). The improvement was also evident in individuals with a CPS ≥1 (14.0 versus 11.3 months; HR: 0.77; 99.3% CI: 0.64–0.92; p<0.0001). Across the study population, OS and PFS still improved for immunotherapy plus chemotherapy compared with chemotherapy alone (median OS: 13.8 versus 11.6 months; HR: 0.80; 95% CI: 0.68–0.94). The results of CheckMate 649 led to the FDA approval of nivolumab in treating advanced or metastatic gastric, GEJ, and oesophageal adenocarcinomas regardless of PD-L1 expression.89
The ATTRACTION-4 trial (ONO-4538 phase II/III, multicentre, randomized study in patients with unresectable advanced or recurrent gastric cancer; ClinicalTrials.gov identifier: NCT02746796) investigated the benefit of adding immunotherapy to chemotherapy as first-line therapy in advanced gastric or GEJ adenocarcinoma unselected for PD-L1 overexpression or dMMR/MSI-H status.90,91 The study enrolled 724 Asian patients and randomly assigned them to treatment with either nivolumab or placebo combined with oxaliplatin-based chemotherapy. The addition of nivolumab compared with chemotherapy alone resulted in an increased median PFS (10.45 versus 8.34 months; HR: 0.68; 95% CI: 0.51–0.90; p=0.0007), but no OS benefit (17.45 versus 17.15 months; HR: 0.90; 95% CI: 0.75–1.08; p=0.26). Notably, individuals receiving nivolumab plus chemotherapy showed a greater increased ORR (58%) than those getting chemotherapy alone (48%); however, there were also increased treatment-related side effects (58% versus 49%). The trial also failed to demonstrate an OS benefit in patients with PD-L1 expression. However, it was noted that the trial used tumour proportion score (TPS) rather than the CPS; TPS is considered less predictive for upper gastrointestinal adenocarcinomas compared with CPS.
Nivolumab has also been shown to be beneficial in oesophageal SCC when treated in combination with chemotherapy or as monotherapy. Multiple studies have found evidence for the combination of nivolumab and chemotherapy in the frontline setting in patients with SCC.85,92,93 In the Checkmate 648 trial (A randomized phase III study of nivolumab plus ipilimumab or nivolumab combined with fluorouracil plus cisplatin versus fluorouracil plus cisplatin in subjects with unresectable advanced, recurrent or metastatic previously untreated esophageal squamous cell carcinoma; ClinicalTrials.gov identifier: NCT03143153), patients with previously untreated metastatic oesophageal SCC were randomly assigned to nivolumab plus fluorouracil and cisplatin (chemotherapy), nivolumab plus ipilimumab or chemotherapy alone, irrespective of PD-L1 expression.92,94 Total survival improved significantly in the group who got nivolumab with chemotherapy versus chemotherapy alone, both in the overall cohort and in those with PD-L1 expression >1 (overall cohort: 13.2 versus 10.7 months; HR: 0.74; 95% CI: 0.58–0.96. PD-L1 expression >1: 15.4 versus 9.1 months; HR: 0.54; 95% CI: 0.37–0.80).
In the phase III ATTRACTION-3 trial (ONO-4538 phase III, multicentre, randomized, open-label study in patients with oesophageal cancer refractory or intolerant to combination therapy with fluoropyrimidine and platinum-based drugs; ClinicalTrials.gov identifier: NCT02569242), 419 patients with advanced oesophageal SCC were randomized to either nivolumab or chemotherapy (paclitaxel or docetaxel), irrespective of PD-L1 expression.95,96 The median OS was improved in the nivolumab group compared with chemotherapy (10.9 versus 8.4 months; HR for death: 0.77; 95% CI: 0.62–0.96). The added benefit of nivolumab was seen in all patients who received nivolumab in the subgroup analysis, irrespective of PD-L1 expression. The study results led to the FDA approval of nivolumab monotherapy in patients with metastatic oesophageal SCC who progressed on fluoropyrimidine- and
platinum-based chemotherapy.
The combination of PD-L1 and cytotoxic T-lymphocyte-associated antigen 4 therapy has also been explored in GECs, given the efficacy of nivolumab and ipilimumab in treating other solid tumours, including renal cell carcinoma and melanomas.97,98 The CheckMate 649 trial further evaluated first-line nivolumab plus chemotherapy versus chemotherapy alone and nivolumab plus ipilimumab versus chemotherapy alone in patients with metastatic gastric, oesophageal or GEJ adenocarcinoma. Patients with PD-L1 CPS ≥5 who received ipilimumab and nivolumab in combination showed no survival benefit compared with chemotherapy alone (median OS: 11.2 versus 11.6 months; HR: 0.89; p=0.2302).87
The CheckMate 648 trial only enrolled patients with SCC histology. The nivolumab/ipilimumab combination improved OS, compared with chemotherapy alone (12.8 versus 10.7 months; HR: 0.78; 95% CI: 0.62–0.98).92 Meanwhile, the nivolumab/ipilimumab group experienced more adverse effects than the nivolumab plus chemotherapy group, resulting in more treatment termination (47% versus 34%). Nivolumab with chemotherapy showed improved OS and PFS, as well as a better adverse effect profile than nivolumab/ipilimumab.
Ipilimumab has not been found to improve survival when used as monotherapy. A phase II study was conducted with 114 patients with advanced gastric or GEJ adenocarcinomas that had progressed following fluorine- and platinum-based chemotherapy.99 The study randomized the patients to receive ipilimumab or BSC. Unfortunately, the study failed to meet either the primary endpoint of immune-related PFS (2.92 versus 4.90 months; HR: 1.44; 80% CI; 1.09–1.91; p=0.097) or the OS benefit (12.7 versus 12.1 months) in both the ipilimumab and the BSC arm.
A list of clinical trials that have used immunotherapy is shown in Table 3.77,78,80,83–88,90–92,94–96,100–105
Conclusions
Recent advances in molecular profiling have helped improve the knowledge of advanced GEC’s genomic and epigenomic status. These advancements have identified many genetic mutations, chromosomal abnormalities, and transcriptional and epigenetic changes; furthermore, they have opened the door to tailored therapy options for individuals with advanced GEC. Despite all these efforts, only a few targeted therapies have been shown to be effective. Several biomarker-based clinical studies have failed to demonstrate a survival benefit; the reason for this failure is not fully understood. In addition to the high degree of genomic and phenotypic heterogeneity even within individual tumours, intrinsic and acquired resistance mechanisms, such as those found with anti-HER2 therapies, are major reasons for such failures.14,106
The need to use comprehensive molecular profiling widely, a deeper understanding of the tumour microenvironment, and less invasive approaches during treatment by using circulating tumour DNA to identify targetable mutations and characterize clones of resistance will aid the field efforts to better personalize therapies.107 We believe that these on-going efforts will better serve the enrolment of patients into clinical trials to incorporate targeted and immunotherapies in metastatic settings and neoadjuvant and adjuvant settings to help improve outcomes for patients with GEC.












