Cholangiocarcinoma (CCA) is a cancer of the epithelial cells lining the biliary tree in the liver and can occur both inside the liver (intrahepatic CCA [iCCA]) and outside the liver (extrahepatic CCA, which includes both perihilar and distal cholangiocarcinoma) (Figure 1).
Figure 1: A diagram showing the anatomical locations of the different subtypes of biliary tract cancer and the approximate total percentage of incidence of each subtype out of all biliary tract cancers. Extrahepatic cholangiocarcinoma includes both perihilar and distal cholangiocarcinoma.
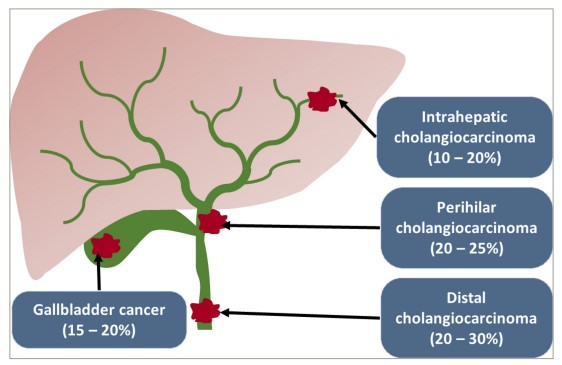
Reproduced and adapted with permission from Banales et al.1(http://creativecommons.org/licenses/by/4.0/)
While CCA is a relatively rare cancer globally, it is the second most common hepatobiliary cancer after hepatocellular carcinoma and accounts for 3–5% of gastrointestinal cancers worldwide, with the incidence of iCCA rising over the past few decades.1–4 The current first-line standard-of-care treatment for patients with unresectable, locally advanced or metastatic CCA is cisplatin and gemcitabine chemotherapy, with the addition of durvalumab if available.5,6 Second-line treatment is with folinic acid, fluorouracil and oxaliplatin (FOLFOX) chemotherapy; however, outcomes are poor, with the overall survival of patients being less than 1 year and the benefit of second-line FOLFOX chemotherapy being around 1 month when compared with best supportive care.5,7 There is, therefore, an urgent need for new and more effective therapies for patients with advanced CCA.
A major focus of investigation in the treatment of CCA is the use of targeted systemic anti-cancer therapies. iCCAs have a wide range of actionable genetic alterations, including receptor tyrosine-protein kinase erbB-2 (ERBB2) amplifications, isocitrate dehydrogenase 1 (IDH1) mutations and fibroblast growth factor receptor 2 (FGFR2) fusions.8,9 Fibroblast growth factors (FGFs) and their receptors (FGFRs) are molecules involved in a variety of cell signalling pathways, which control cell functions such as proliferation, survival, migration and differentiation.10 There are four FGFRs (FGFR1, FGFR2, FGFR3 and FGFR4), all of which are receptor tyrosine kinases (RTKs) consisting of an extracellular ligand-binding domain and an intracellular tyrosine-kinase domain.11 As FGFRs have major effects on cell proliferation and survival, it is unsurprising that mutations, fusions and amplifications of FGFRs are relatively common alterations found in malignant cells. Around 5–10% of all human cancers have an FGFR alteration, and alterations in FGFR have been found in multiple cancer types, including breast cancer, non-small-cell lung cancer, gastric cancer and squamous cell head and neck cancer.12–19
Around 15–20% of iCCAs have FGFR2 gene fusions, so targeting FGFR2 fusions in CCA has been a major research focus.20 This article focuses on futibatinib, which is a second-generation FGFR inhibitor, and its efficacy and safety in patients with previously treated, unresectable, locally advanced or metastatic iCCA.
Fibroblast growth factor receptor 2 (FGFR2) inhibitors in cholangiocarcinoma
Futibatinib (TAS-120, Lytgobi® [Taiho Pharmaceutical Co., Ltd., Japan]) is a selective inhibitor of FGFR1–4 and binds covalently and irreversibly to a conserved cysteine residue in the P-loop of the FGFR kinase domain.21,22 This is a different mode of action from other FGFR inhibitors, such as pemigatinib and infigratinib, which are reversible, FGFR 1–3 selective and inhibit FGFR function by adenosine triphosphate (ATP)-competitive binding.23–25 Both pemigatinib and infigratinib have been approved by the US Food and Drug Administration (FDA) for the treatment of patients with previously treated, unresectable, locally advanced or metastatic CCA with detectable FGFR2 fusions based on the results of two phase II clinical trials (A phase 2, open-label, single-arm, multicenter study to evaluate the efficacy and safety of pemigatinib in subjects with advanced/metastatic or surgically unresectable cholangiocarcinoma including FGFR2 translocations who failed previous therapy – [FIGHT-202], ClinicalTrials.gov identifier: NCT02924376 and A phase II multicenter, single arm study of oral BGJ398 in adult patients with advanced or metastatic cholangiocarcinoma with FGFR2 gene fusions or other FGFR genetic alterations who failed or are intolerant to platinum-based chemotherapy, ClinicalTrials.gov identifier: NCT02150967) demonstrating response rates of 35.5 and 23.1%, respectively.23,24
However, a major issue with FGFR2 inhibitor therapy, as with all RTK-targeted small-molecule inhibitors, is the development of acquired resistance to treatment.26 A study investigating three patients who had developed secondary resistance to infigratinib, showed that all three had developed FGFR2 kinase domain mutations, preventing infigratinib binding.27 Since futibatinib binds irreversibly to an alternate site on the FGFR2 kinase, it is less likely to cause on-target acquired resistance than infigratinib and pemigatinib.22,28 This was demonstrated in an in-vitro study showing that futibatinib treatment caused the development of fewer acquired drug resistance mutations than ATP-competitive FGFR inhibitors.29 This has led to futibatinib being investigated in clinical trials for patients with FGFR2 fusions and advanced iCCA, both in the second-line setting after treatment with chemotherapy and after the development of acquired resistance to other FGFR2 inhibitor treatments (Table 1).20,23,30–47
Table 1: Comparison of the fibroblast growth factor receptor inhibitors currently in use for the treatment of cholangiocarcinoma20,23,30–47
|
|
FGFR inhibitor |
||
|
|
Futibatinib |
Pemigatinib |
Infigratinib |
|
Lytgobi® (TAS-120; Taiho Pharmaceutical Co., Ltd., Japan) |
Pemazyre® (INCB054828; Incyte Biosciences Distribution B.V., The Netherlands) |
Truseltiq® / Febseltiq® (BGJ-398; QED Therapeutics [BridgeBio Pharma], USA) |
|
|
FGFR targets |
FGFR1-4 |
FGFR1-3 |
FGFR1-3 |
|
Type of binding |
Irreversible |
Reversible |
Reversible |
|
Current clinical trials |
Ongoing phase III clinical trial in first line (FOENIX-CCA3 [TAS-120-301,; ClinicalTrials.gov identifier: NCT04093362]) and second line (FOENIX-CCA2 [TAS-120-101, ClinicalTrials.gov identifier: NCT02052778] and FOENIX-CCA4 [ClinicalTrials.gov identifier: NCT05727176]) |
Ongoing phase III clinical trial (FIGHT-302; [ClinicalTrials.gov identifier: NCT03656536]) |
Phase III trial (PROOF-301; [ClinicalTrials.gov identifier: NCT03773302]) discontinued |
|
Current licencing status |
Licenced by the FDA for adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma harbouring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements. |
Licenced by the FDA for or the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test. |
Licenced by FDA for adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test. |
|
|
|
|
|
|
Conditional marketing authorisation has been granted by the EMA for futibatinib in the second-line treatment of locally advanced or metastatic cholangiocarcinoma characterised by fusion or rearrangements of fibroblast growth factor receptor (FGFR). |
Licenced by the EMA for the second-line treatment of locally advanced or metastatic cholangiocarcinoma characterised by fusion or rearrangements of fibroblast growth factor receptor 2 (FGFR2). |
Application for licensing has been withdrawn from the EMA and NICE. |
|
|
|
|
|
|
|
Recommended for approval by NICE. |
Licenced by NICE as an option for treating locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or rearrangement that has progressed after systemic therapy in adults. |
|
|
|
Efficacy |
From the FOENIX-CCA2 (ClinicalTrials.gov identifier: NCT02052778) single arm, open label phase II clinical trial. Overall response rate of 42 % (95% CI 32–52%) with a complete response rate of 0.9 %. |
From the single arm, open label FIGHT-202 (ClinicalTrials.gov identifier: NCT02924376), a single-arm phase II clinical trial. Overall response rate of 37.0 % (95% CI 27.94, 46.86), with a complete response rate of 2.8 %. |
From the single arm, open label CBGJ398×2204 (ClinicalTrials.gov identifier: NCT02150967) clinical trial. Overall response rate of 23.1 % (95% CI 15.6 – 32.2) with a complete response rate of 0.9 %. |
|
|
|
|
|
|
The median progression free survival was 9.0 months (95% CI 6.9 – 13.1) and median overall survival was 21.7 months (95% CI 14.5 – not reached), with a median duration of response of 9.7 months (95% CI 7.6 – 17.0) |
For patients with FGFR2 rearrangements, the median progression free survival was 7.0 months (95% CI 6.1 – 10.5) and median overall survival was 17.5 months (95% CI 14.4 – 22.9), with a median duration of response of 9.1 months (95% CI 6.0 – 14.5) |
The median progression free survival was 7.29 months (95% CI 5.59 – 7.56) and median overall survival was 11.86 months (95% CI 10.68 – 14.85), with a median duration of response to 5.55 months (95% CI 3.78 – 7.66) |
|
|
|
|
|
|
|
Main toxicities |
From TAS-120-101 (FOENIX-101, ClinicalTrials.gov identifier: NCT02052778), the most common (≥20%) toxicities were hyperphosphataemia (89.7%), nail disorders |
Hyperphosphataemia (60.5 %), alopecia (49.7 %), diarrhoea (47.6 %), nail toxicity (44.9 %), fatigue (43.5 %), nausea (41.5 %), stomatitis (38.1 %), constipation (36.7 %), dysgeusia (36.1 %), dry mouth (34.0 %), arthralgia (29.9 %), dry eye (27.9 %), hypophosphataemia (23.8 %), dry skin (21.8 %), and palmar-plantar erythrodysaesthesia syndrome (16.3 %). |
Hyperphosphataemia (76.9), stomatitis (54.6), fatigue (40.7), alopecia (39.8), dry eye (36.1), arthralgia |
|
(44.1%), constipation (37.2%), alopecia (35.2%), diarrhoea (33.8%), dry mouth (31.0%), fatigue |
|
(32.4), dysgeusia (31.5%), constipation (31.5%), dry mouth (25.9%), diarrhoea (25.0%), decreased |
|
|
(31.0%), nausea (28.3%), dry skin (27.6%), increased AST (26.9%), abdominal pain (24.8%), |
Serous retinal detachment occurred in 4.8 % of patients, but was only ≥ Grade 3 in 0.7 % of patients. |
appetite (24.1%), dry skin (24.1%), hypophosphataemia (23.1%), vomiting (21.3%), nausea (19.4%), |
|
|
stomatitis (24.8%), vomiting (23.4%), palmar-plantar erythrodysaesthesia syndrome (22.8%), |
|
abdominal pain (16.7%) pain in extremity (15.7%) and back pain (14.8%). |
|
|
arthralgia (21.4%), and decreased appetite (20.0%). |
|
|
|
|
Discontinuation rate due to toxicities |
The most common serious adverse reactions included were intestinal obstruction (1.4%) and migraine (1.4%). |
Serious adverse reactions included hyponatraemia (2.0 %), one episode of which (0.7%) led to dose interruption, and blood creatinine increase (1.4 %), one episode of which led to dose discontinuation (0.7%). |
The most common serious (grade three or 4) adverse reactions included hyperphosphataemia (7.7%) and stomatitis (5.8%), with grade four increased lipase (0.8%) and hypophosphataemia (0.6%) also seen. |
|
|
|
|
|
|
7.6 % of patients discontinued futibatinib due to adverse reactions; the most common was stomatitis (1.4%) while all other reactions leading to discontinuation occurred in single patients. |
No episodes of hyperphosphataemia or retinal detachment led to dose discontinuation. |
19 % of patients discontinued treatment due to adverse events. |
|
|
Dosing and dose reductions |
Starting dose is 20 mg taken orally once daily. |
Starting dose is 13.5 mg taken once daily for 14 consecutive days, followed by 7 days off therapy. First dose reduction is to 9 mg once daily for 14 days, followed by 7 days off therapy, second dose reduction is to 4.5 mg once daily for 14 days followed by 7 days off therapy. |
Starting dose is 125 mg once daily for 21 consecutive days followed by 7 days off therapy. First dose reduction is 75 mg once daily for 21 days followed by 7 days of therapy, second dose reduction is to 50 mg once daily for 21 days followed by 7 days off therapy. |
|
First dose rection is to 16 mg once daily, second dose reduction is to 12 mg once daily. |
|||
|
Clinical particulars |
Dietary restrictions to limit phosphate intake are recommended as part of hyperphosphataemia management. |
Ophthalmological examination before starting treatment and every 2 months for the first 6 months of treatment, every 3 months afterwards, and urgently at any time for visual symptoms. |
Ophthalmological examination before starting treatment, at 1 month, 3 months and then every 3 months during treatment. |
|
|
|||
|
Ophthalmological examination should be performed before starting treatment. |
|||
Information on different FGFR inhibitors has been taken from references.20,23,30–47
AST = aspartate transferase ;CI = confidence interval;EMA = European Medicines Agency;FDA = US Food and Drug Administration;FGFR2 = fibroblast growth factor receptor 2;NICE = National Institute for Health and Care Excellence.
Phase I clinical trials using futibatinib
The first-in-human, phase I, dose-escalation clinical trial of futibatinib (Phase 1/2 study of TAS-120 in patients with advanced solid tumors harboring FGF/FGFR aberrations [FOENIX-101]; ClinicalTrials.gov identifier: NCT02052778) included 86 patients, of whom 24 (28%) had CCA and 71 (83%) had FGFR aberrations.48 This study showed that futibatinib had tolerable adverse effects (Table 1) and recommended a dose of 20 mg once per day (q.d.). Partial responses were recorded in 5 patients, of whom 2 had FGFR2-fusion-positive iCCA, and stable disease in 41 other patients (48%).48 The encouraging results of this trial led to the phase I, dose-expansion part of FOENIX-101 in patients with FGFR alterations, which recruited 197 patients with advanced solid tumours of varying types.25 Overall, 37.6% of the patients had CCA, and the majority of these (61/64, 95.3%) had iCCA. Most of the cohort had FGFR fusions (85/170, 50.0%), with FGFR mutations (51/170, 30.0%), FGFR amplifications (24/170; 14.1%) and FGF1/3/4/19 ligand amplifications (23/170; 13.5%) also common in the cohort.25 Most patients were heavily pre-treated, with the majority (75.3%) having received at least two prior regimens, and 33 patients (19.4%) having received previous FGFR inhibitor therapy.25 Futibatinib generally showed good efficacy in this trial, with more than half of the patients (103/197, 52.3%) having some tumour shrinkage. Patients with CCA did particularly well, with a higher response rate than any other tumour type. The overall response rate was 15.6% among patients receiving a higher futibatinib dose (20 mg), and the disease control rate was 71.9%, with a median duration of response of 5.3 months.25 Even in patients who had previously received FGFR inhibitors, 17.9% had an objective response despite having had to stop treatment with other FGFR inhibitors due to disease progression .
Phase II clinical trials using futibatinib
Due to the promising results of phase I clinical trials, particularly in patients with FGFR-fusion-positive iCCA previously treated with other FGFR inhibitors, the FOENIX-CCA2 (Phase 1/2 study of TAS-120 in patients with advanced solid tumors harboring FGF/FGFR aberrations; ClinicalTrials.gov identifier: NCT02052778) clinical trial was launched.28,49 This was a multinational, single-arm, phase II study recruiting patients with unresectable or metastatic iCCA with an identified FGFR fusion or rearrangement, who had received at least one prior line of treatment (including at least one line of gemcitabine and cisplatin) but had not received previous treatment with an FGFR inhibitor.28 A total of 103 patients were enrolled, who received at least one dose of futibatinib, and 43 (42%) had a response, including one patient having a complete response.28 Overall, 85 of 103 (83%) patients had disease control, and the median duration of response was 9.7 months, with six patients having a response lasting at least 12 months.28 The median progression-free survival was 9.0 months (95% confidence interval [CI], 6.9–13.1), and the overall median survival was 21.7 months (95% CI, 14.5 to not reached), with a 12-month overall survival rate of 72% (95% CI, 62–80).28 While all patients had at least one adverse event, the majority of these were mild, with the most frequent treatment-related adverse events being hyperphosphataemia (85%), alopecia (33%), dry mouth (30%), diarrhoea (28%), dry skin (27%) and fatigue (25%).28 Toxic nail effects were seen in 47% and retinal disorders in 8%, but overall, only 10 patients (10%) had serious adverse events. Fifty-two patients (50%) had dose interruptions due to adverse events, and 56 (54%) required dose reductions, but only 2 patients (2%) permanently discontinued futibatinib due to treatment-related adverse events.28 The quality of life of patients was also maintained throughout the trial, with nearly all European Organisation for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30) scores remaining stable throughout treatment with futibatinib.50
A further phase II clinical trial using futibatinib, FOENIX-CCA4 (Phase 2 study of futibatinib 20 mg and 16 mg in patients with advanced cholangiocarcinoma with FGFR2 fusions or rearrangements; ClinicalTrials.gov identifier: NCT05727176), is also in progress.30 This an open-label randomized trial recruiting 120 patients to compare the safety and efficacy of 20 mg versus 16 mg dosage of futibatinib in patients with locally advanced, unresectable or metastatic CCA who have previously received cisplatin and gemcitabine chemotherapy. This trial does not include patients who have previously received treatment with an FGFR inhibitor, but will help to clarify whether 16 or 20 mg should be the recommended dose of futibatinib going forward.30
Phase III clinical trials using futibatinib
While the FOENIX-CCA2 trial has now been completed, there are several ongoing and upcoming clinical trials for futibatinib in advanced iCCA; for example, an expanded-access trial is currently ongoing, so patients could receive futibatinib before it is fully approved (An open-label expanded access program of futibatinib (TAS-120) in patients with advanced cholangiocarcinoma harboring FGFR2 gene rearrangements; ClinicalTrials.gov identifier: NCT04507503).51 However, there are two upcoming and ongoing phase III clinical trials for futibatinib in advanced biliary tract cancer. The upcoming trial is SAFIR-ABC10 (Molecular targeted maintenance therapy versus standard of care in advanced biliary cancer: An international, randomised, controlled, open-label, platform phase 3 trial; ClinicalTrials.gov identifier: NCT05615818), which aims to use personalized medicine in the first-line treatment of patients with advanced biliary tract cancers.31,52,53 In this trial, all 800 patients with newly diagnosed locally advanced or metastatic biliary tract cancer will receive four cycles of durvalumab, gemcitabine and cisplatin treatment, as per the current standard of care. All patients will be molecularly profiled using FoundationOne (Foundation Medicine, Cambridge, MA, USA), and patients will be randomized 2:1 to receive either a maintenance treatment based on the presence of any molecular aberrations, or continued durvaulmab, gemcitabine and cisplatin. For patients with FGFR2 fusions, the molecularly targeted treatment used will be futibatinib.
Another phase III trial using futibatinib is the FOENIX-CCA3 (A phase 3, open-label, randomized study of futibatinib versus gemcitabine-cisplatin chemotherapy as first-line treatment of patients with advanced cholangiocarcinoma harboring FGFR2 gene rearrangements; ClinicalTrials.gov identifier: NCT04093362) clinical trial.32,54 Unlike other clinical trials where futibatinib is being used after a patient receives first-line chemotherapy, this trial aims to evaluate futibatinib in the first-line setting. A total of 216 patients with metastatic or unresectable iCCA which harbours FGFR2 rearrangements will be enrolled and randomized 1:1 to receive either 20 mg futibatinib daily until disease progression, or gemcitabine and cisplatin chemotherapy for either 8 cycles or until disease progression.32
Licensing and approval of futibatinib
Based on the results of the phase II FOENIX-CCA2 clinical trial, futibatinib received accelerated approval by the FDA for the treatment of adult patients with previously treated, unresectable, locally advanced or metastatic iCCA with FGFR2 gene fusions or other rearrangements on 30 September 2022.55 The European Medicines Agency (EMA) has also recommended conditional marketing authorization for futibatinib in the same patient cohort as of April 2023, and the decision on approval is still awaited.56 The UK National Institute for Health and Care Excellence (NICE) is also considering the licensing of futbatinib, with a decision expected in September 2024.57
Resistance to futibatinib and alternative FGFR inhibitors
While futibatinib is now licensed in the USA and is under investigation for licensing by other regulatory bodies such as the EMA and NICE, it is not the only novel FGFR inhibitor currently under investigation. One of the major benefits of futibatinib is that it has efficacy in patients with primary resistance to other FGFR inhibitors and who have developed acquired secondary resistance after treatment with other FGFR inhibitors (Table 2).58
Table 2: Results of preclinical experiments in mouse Ba/F3 cells investigating the activity of FGFR inhibitors against various acquired mutations in the FGFR2 kinase domain
|
FGFR2 mutation |
Kinase domain region |
Factor change in IC50 versus wild-type FGFR2 |
||
|
|
|
Futibatinib |
Pemigatinib |
Infigratinib |
|
Wild type |
- |
1 |
1 |
1 |
|
N550D |
Regulatory triad |
2 |
102 |
81 |
|
N550K |
Regulatory triad |
8 |
164 |
68 |
|
V563L |
- |
3 |
5 |
14 |
|
V565I |
Gatekeeper |
4 |
42 |
>236 |
|
V565L |
Gatekeeper |
44 |
335 |
>236 |
|
E566A |
Regulatory triad |
3 |
8 |
12 |
|
E566G |
Regulatory triad |
2 |
6 |
10 |
|
K642I |
Regulatory triad |
2 |
20 |
15 |
|
K642R |
Regulatory triad |
2 |
7 |
16 |
|
K660M |
Activation loop |
5 |
23 |
63 |
FGFR inhibition was assessed by growth suppression of cells, with green, orange and red representing the changes in attenuation by a factor of <5, 5−10 and >10, respectively, in the IC50 compared with the inhibition of wild-type FGFR2.
From The New England Journal of Medicine, Lipika Goyal, Funda Meric-Bernstam, Antoine Hollebecque, et al, Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma, 388, 228–239. Copyright © (2024) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. 28
FGFR = fibroblast growth factor receptor;IC50 = half-maximal inhibitory concentration.
However, there are still gatekeeper mutations that block the efficacy of futibatinib, the most common of which include V565F and V565L mutations in the FGFR2 gene.28,58 Both mutations alter the binding domain of FGFR2, causing a steric clash and preventing the binding of futibatinib (and other FGFR inhibitors) into the ATP-binding pocket of FGFR2.58 While research into the development of resistance to FGFR inhibitors is ongoing, case studies indicate that gatekeeper mutations can develop rapidly with treatment with FGFR inhibitors, and although they develop more slowly with futibatinib than alternatives, they still prove to be a major issue with this treatment (Figure 2).28,58,59
Figure 2: This diagram shows the predicted interaction of futibatinib with the ATP-binding pocket of the FGFR2 wild-type kinase domain59
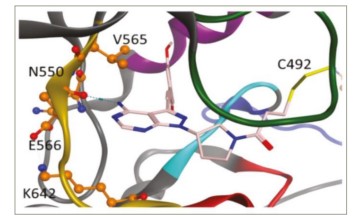
The amino acid residues of the identified mutations causing the FGFR inhibitor resistance are labelled and depicted using ball-and-stick models.
From The New England Journal of Medicine, Lipika Goyal, Funda Meric-Bernstam, Antoine Hollebecque, et al, Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma, 388, 228-239. Copyright © (2024) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.28
However, a new generation of FGFR inhibitors is under development to overcome this issue, one of which is RLY-4008 (lirafugratinib [Relay Therapeutics, USA]). RLY-4008 potently and selectively targets primary oncogenic FGFR2 alterations, including those that develop due to acquired resistance to other pan-FGFR inhibitors, including futibatinib.60 REFOCUS (A first-in-human study of highly selective FGFR2 inhibitor, RLY-4008, in patients with intrahepatic cholangiocarcinoma (ICC) and other advanced solid tumors; ClinicalTrials.gov identifier: NCT04526106), a phase I/II, dose-escalation, first-in-human clinical trial in patients with unresectable or metastatic iCCA and other solid tumours, is currently ongoing.60–62 The current results from the initial phase I trial demonstrate that RLY-4008 has good efficacy in patients, with tumour reduction seen in 64% of patients and all four patients with FGFR-naive CCA responding to treatment at the targeted dose.61 Responses were also seen in patients previously treated with FGFR inhibitors, and the side effects were tolerable. The phase II part of REFOCUS is currently recruiting and ongoing.61
Tinengotinib (TT-00420 [TransThera Sciences (Nanjing), Inc., China]), a pan-tyrosine kinase inhibitor (TKI) that inhibits FGFR1–3, vascular endothelial growth factor receptor (VEGFR), colony-stimulating factor-1 receptor, aurora kinase and platelet-derived growth factor receptor (PDGFR), is also under investigation for the treatment of metastatic CCA. A phase II clinical trial (P-77,TT420C1206; A phase II, open label, multicenter study to evaluate the efficacy and safety of TT-00420 (tinengotinib) tablet in adult patients with advanced cholangiocarcinoma; ClinicalTrials.gov identifier: NCT04919642) showed 90% disease control (complete response or partial response plus stable disease) in patients with FGFR2 fusions or rearrangements, with tolerable side effects.63,64 The majority (93.3%) had received at least one previous line of treatment with an FGFR inhibitor, indicating that tinengotinib may also be a strong option for patients with resistance to other FGFR inhibitors. A phase III clinical trial (FIRST-308, TT420C2308; A phase III, randomized, controlled, global multicenter study to evaluate the efficacy and safety of oral tinengotinib vs physician’s choice in subjects with FGFR-altered, chemotherapy- and FGFR inhibitor-cholangiocarcinoma; ClinicalTrials.gov identifier: NCT05948475) is due to start recruiting patients with FGFR-altered and chemotherapy- and FGFR inhibitor-refractory or relapsed CCA, randomized between tinengotinib and physician’s choice to investigate the efficacy and safety of this drug in more detail.65,66
Perspectives
Futibatinib has been shown to have good efficacy, with tolerable side effects, in a tumour type where outcomes are poor and there are few treatment options. Although it is only licensed so far by the FDA, it is likely to be licensed soon by other major regulatory authorities. Pemigatinib is already licensed, and while there have not been any studies to directly compare different FGFR inhibitors, a study carrying out an indirect comparison showed that futibatinib provided longer survival versus chemotherapy than other FGFR inhibitors.33 Futibatinib also has the benefit of being effective in patients who have primary resistance to other FGFR inhibitors or have acquired resistance after treatment with other FGFR inhibitors, a group who at present lack effective treatment options. The results of the upcoming and ongoing SAFIR-ABC-10 and FOENIX-CCA3 clinical trials will also be important to determine the efficacy of first-line FGFR inhibitor treatment and whether it is a good alternative to chemotherapy. At present, less than half of patients with non-resectable CCA at presentation receive systemic anti-cancer therapy, most commonly due to poor performance status.67 Combination gemcitabine and cisplatin chemotherapy, the current first-line standard of care, can be hard to tolerate for patients with worse performance status; so, if targeted treatments such as futibatinib show good efficacy in this setting, it may prove to be a potential treatment option for those with nonresectable or metastatic CCA with FGFR2 fusions who are not fit for first-line chemotherapy and who currently have no available systemic anti-cancer therapies.
It will also be interesting to see if future trials investigate futibatinib in the adjuvant setting; the current standard of care is treatment with capecitabine, as per the BILCAP clinical trial (A randomised clinical trial evaluating adjuvant chemotherapy with capecitabine compared to expectant treatment alone (observation) following surgery for biliary tract cancer; ClinicalTrials.gov identifier: NCT00363584), or S-1, as per the recent JCOG1202: ASCOT clinical trial (A phase III trial of S-1 vs. observation in patients with resected biliary tract cancer ASCOT: Adjuvant S-1 for cholangiocarcinoma trial (JCOG1202, ASCOT); University Hospital Medical Information Network Clinical Trials Registry identifier: UMIN000011688).68,69 Potentially, an effective FGFR2 inhibitor, such as futibatinib, could prove to be an alternative for FGFR2-fusion-positive patients who otherwise may not be able to tolerate adjuvant chemotherapy and help to reduce the high rates of relapse after surgery.
However, there are still barriers to the use of futibatinib in iCCA. While access to molecular testing for CCA is becoming more and more widespread, issues with a lack of tissue for testing from biopsies and access to the tests themselves prevent some patients from having FGFR2 fusions detected, without which they cannot receive futibatinib. There is still controversy over the best way to test for FGFR fusions, with the main options being either RNA or DNA from tissues or testing of circulating tumour DNA (ctDNA). While ctDNA is easier to obtain from patients than tissue RNA, testing ctDNA may be significantly less sensitive than testing tissue RNA. DNA testing from tissues is also easier than RNA-based testing due to the increased stability of DNA compared with RNA, but DNA-based testing can more easily miss novel fusions, especially those involving large intronic regions.70 This can lead to significant differences in the proportion of patients observed to have FGFR2 fusions; for example, while FGFR2 fusions are generally observed to have a prevalence of around 14% in iCCA, sensitive RNA-based assays have estimated the prevalence to be up to 20.0% in the BILCAP cohort of unselected biliary tract cancer.8,28
Acquired resistance to FGFR inhibitors is less common with futibatinib than with other FGFR inhibitors, but gatekeeper mutations, in particular V565L and V565F, still eventually develop in many patients and cause tumours in patients to progress. The next generation of FGFR inhibitors, such as RLY-4008, may help to treat these patients or prevent the development of these mutations, and it will be interesting to see the results of their upcoming clinical trials and whether these new medications can prove to be a good option for patients who have resistance to futibatinib or other FGFR inhibitors.
FGFR inhibitors, including futibatinib, have significant toxicities, with a relatively high rate of adverse events and discontinuation due to these adverse events. The toxicities of FGFR inhibitors, in particular high rates of hyperphosphataemia and risks to sight from serous retinal detachment, significantly limit the maximum tolerated dose of these medications (see Table 1).20,23,30–47 Having a relatively low maximum tolerated dose may mean that patients with tumours are relatively underdosed with the medication, leading to the more rapid development of resistance to treatment. This may be one reason why the duration of response to most FGFR inhibitors (around 9–12 months) is lower than that to other tyrosine kinase inhibitors with other targets, which often have durations of response around 12–20 months. It will be interesting to see if the newest generation of FGFR inhibitors can reduce the level of toxicity, leading to fewer adverse events, less discontinuation or treatment breaks due to adverse events and potentially a higher duration of response.
In the near future, as more molecular targets are identified in CCA, including ERBB2 amplification, mouse double minute 2 (MDM2) amplification and IDH1 mutations, new medications to target these will no doubt soon be licensed by the FDA, EMA and NICE.8 Together with the results of the TOPAZ-1 (A phase III randomized, double-blind placebo controlled, multi-regional, international study of durvalumab in combination with gemcitabine plus cisplatin versus placebo in combination with gemcitabine plus cisplatin for patients with first-line advanced biliary tract cancers; ClinicalTrials.gov identifier: NCT03875235) phase III clinical trial indicating a potential benefit of upfront immunotherapy with chemotherapy, there will soon be more and more options available for treating patients with iCCA.6,71 Investigating combination treatment with futibatinib and chemotherapy or immunotherapy or other targeted therapy may be one way to increase response rates and the duration of response.
Futibatinib is an effective treatment for patients with unresectable, locally advanced or metastatic iCCAs with FGFR2 fusions and is particularly useful to patients with intrinsic or acquired resistance to other FGFR inhibitors. It is likely to become used more widely in these indications; however, it will be interesting to see how it will be compared with the new generation of upcoming FGFR inhibitors.












