Biliary tract cancers (BTCs) are a heterogeneous group of adenocarcinomas that originate from the epithelial lining of the biliary tree and are classified into cholangiocarcinoma (CCA) and gallbladder cancer. BTC is an aggressive and rare epithelial malignancy that is usually diagnosed at an advanced stage with few effective therapeutic options.1 CCA is divided into extrahepatic and intrahepatic cholangiocarcinoma, with the extrahepatic type further classified into perihilar and distal based on relation to the hilum. Incidence rates have recently increased worldwide; 5–year survival is 5–10% for non-resectable advanced disease and approximately 30% for those treated with curative surgical intent.2,3 The only potentially curative treatment option is surgery; however, only approximately 35% of tumours at diagnosis are amenable to resection.4
Advancements in genomic profiling have revealed critical pathophysiological features of BTCs, leading to the discovery of various actionable molecular targets, including alterations in fibroblast growth factor receptor (FGFR), isocitrate dehydrogenase (IDH), B-Raf proto-oncogene serine/threonine-protein kinase (BRAF), neurotrophic tropomyosin receptor kinase (NTRK) and human epidermal growth factor receptor 2 (HER-2), with development of multiple targeted therapies.5 Over the past few years, the US Food and Drug Administration (FDA) has approved multiple targeted therapies for patients with BTC that has progressed on first–line chemotherapy, including pemigatinib, infigratinib, futibatinib and ivosidenib. However, these targeted agents benefit only a small subset of patients with BTCs that harbour specific actionable alterations. For the majority of patients, chemotherapy remains the only viable option.
In patients with BTCs who have unresectable or metastatic disease, the combination of gemcitabine and cisplatin chemotherapy serves as first–line therapy based on the ABC-02 trial published in 2010.6 The ABC-02 trial demonstrated the efficacy of cisplatin plus gemcitabine with an improved overall survival (OS) compared with single-agent gemcitabine (median OS 11.7 months versus 8.1 months).6 However, progress to improve the first–line therapy options for BTCs remains slow, with gemcitabine plus cisplatin being the standard of care for more than a decade. Only one recently published phase III trial has demonstrated improved outcomes by the addition of durvalumab to gemcitabine plus cisplatin in patients with advanced BTCs (TOPAZ-1, see below). This article reviews the immunotherapeutic options for patients with BTCs, describes the studies that led to the TOPAZ-1 trial, and summarizes key areas of research that are necessary to inform future drug development and improve patient outcomes.
Immune checkpoint inhibitor physiologyImmune Checkpoint Inhibitors Physiology
In addition to small molecule inhibitors, immune checkpoint inhibitors (ICIs) have emerged as a potential option for patients with BTCs. Preclinical studies demonstrated a promising immune microenvironment for ICI usage.7 Initial data demonstrated extensive immune cell involvement in CCA, with improved survival, decreased rates of metastasis and better Tumour-Node-Metastasis staging correlating with the presence of CD4 and CD8 T cells, and poor prognosis associated with macrophage and neutrophil involvement.8,9 Programmed death-ligand 1 (PD-L1) expression is seen in more than 45% of tumours and has been associated with a worse prognosis and poor survival.10,11 Additionally, in a study by Nakamura et al., 40% of the CCA tumours assessed were determined to have a high mutational load and high immune checkpoint expression.12 Furthermore, a pathological study of occupational–associated CCA demonstrated higher levels of PD-L1–expressing lymphocytes and infiltrating CD-8 cells, and hypothesized that these tumours demonstrated immune escape via their expression of PD-L1.13
Based on work in previous cancers, ICIs are known to produce improved responses with various malignancies, especially in those with increased tumour mutational burden (TMB) and high microsatellite instability (MSI-H). Genetic studies of CCA demonstrated a modest prevalence of MSI-H and high TMB. In one large–scale genetic analysis, 2–3.5% of CCA was classified as high TMB with >17 somatic missense mutations per mega base (Mb) analysed.14 In several additional studies, CCA was associated with a moderate burden of MSI-H and deficiency in mismatch repair proteins with a prevalence around 5%.[14″>15,16
Single–agent immunotherapy
Multiple trials have evaluated the use of single–agent immunotherapy in patients with CCA following progression on chemotherapy (Table 1).17–26 Pembrolizumab, a monoclonal antibody that binds to the programmed death 1 (PD-1) receptor has recently been studied in CCA in two trials, KEYNOTE-158 and KEYNOTE-028.17 These were phase II and phase Ib trials that used pembrolizumab in patients with incurable CCA that progressed after standard treatment regimens. The KEYNOTE-028 trial required patients to have PD-L1–positive tumours, whereas the KEYNOTE-158 trial was open to patients regardless of PD-L1 status. The objective response rate (ORR) was 5.8% in KEYNOTE-158 and 13.0% in KEYNOTE-028. The median OS and PFS were 7.4 months and 2.0 months, respectively, in KEYNOTE-158 compared with 5.7 months and 1.8 months, respectively, in KEYNOTE-028. In a subgroup analysis of KEYNOTE-158, the ORR was 6.6% in PD-L1 expressors compared with 2.9% in non–expressors.17
Table 1: Key clinical trials with checkpoint inhibitors
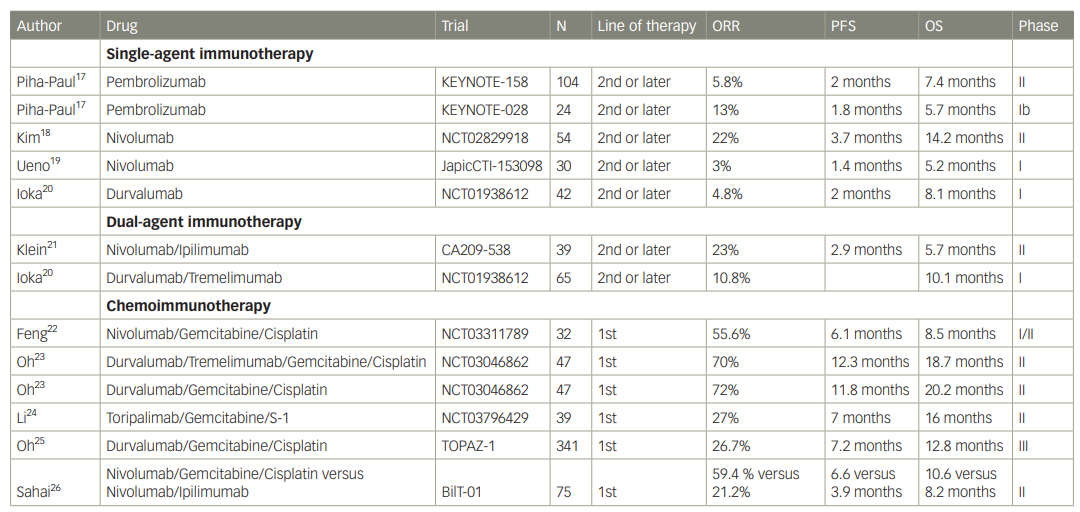
ORR = objective response rate; OS = overall survival; PFS = progression-free survival.
Another PD-1 inhibitor, nivolumab, has been studied as a second–line treatment in patients with CCA. An early phase I trial comparing nivolumab alone versus in combination with gemcitabine plus cisplatin demonstrated improved median OS associated with PD-L1 expression; the single patient with a partial response was found to have deficiency in mismatch repair proteins.19 Kim et al. evaluated single–agent nivolumab in a phase II trial of 54 patients with advanced, refractory BTCs.18 The trial demonstrated median progression-free survival (PFS) and OS of 3.7 months (95% confidence interval [CI]: 2.3–5.7) and 14.2 months (95% CI: 5.9–not reached), respectively, with an ORR of 22%. In patients with PD-L1 expression, there was a superior median PFS compared with PD-L1–negative tumours (10.4 months versus 2.3 months; p<0.001).18 In another phase I trial of 42 patients, treatment with durvalumab, a PD-L1 inhibitor, was associated with median PFS and OS of 2 months and 8.1 months, respectively.20
The above studies suggest that single–agent ICIs have modest activity in patients with BTCs who have progressed on prior chemotherapy. Response rates of 5–20% are noted, with median PFS of about 2 months. Currently, there is no good biomarker to predict the efficacy of single–agent immunotherapy. In one retrospective study of 47 patients with BTCs treated with immunotherapy, patients with TMB >5 mutations/Mb had improved PFS and OS compared with patients with TMB <5 mutations/Mb.27
Dual–agent immunotherapy
Treatment with dual ICIs has been attempted following at least one line of systemic therapy, but thus far has not demonstrated effective responses compared with single–agent ICIs (Table 1).20,21 In a study by Ioka et al., durvalumab and tremelimumab, a CTLA-4 inhibitor, were used as single agents or in combination, and demonstrated no significant differences between the single agent or combination therapy, with median OS of 8.1 versus 10.1 months, respectively, and duration of response of 9.7 versus 8.5 months, respectively.20 The ORR with single–agent durvalumab and the combination of durvalumab plus tremelimumab was 4.8% and 10.8%, respectively. Nivolumab plus ipilimumab combination in a phase II trial demonstrated an ORR of 23% and a disease control rate of 44%.21 Interestingly, there was no evidence of MSI-H in the responding patients and all the responses were seen in patients with intrahepatic CCA, with no response in patients with extrahepatic CCA.21
Chemotherapy plus immunotherapy
As only modest activity was observed with single–agent and dual–agent ICIs in advanced BTCs, the combination of immunotherapy and standard–of–care chemotherapy was evaluated (Table 1).22–26 An initial phase II study examining nivolumab in combination with gemcitabine plus cisplatin demonstrated a PFS of 6.1 months (95% CI: 3.4–8.2) and ORR of 55.6%.22 Treatment–related adverse events were manageable, with only one patient experiencing immune–related adverse events. A phase Ib/II trial evaluated the addition of nivolumab to 5-fluorouracil and liposomal-irinotecan as second–line therapy.28 Median PFS and OS were 4.2 months (95% CI: 1.9-10.2) and 7.5 months (95% CI: 5.8–21.4), respectively, and, according to the authors’ conclusions, this study failed to reject the null hypothesis (H0=PFS of 2.9 months) due to overlapping confidence intervals.
In a phase II trial assessing nivolumab, cisplatin, plus gemcitabine compared with nivolumab plus ipilimumab as first–line treatment for advanced BTCs, there was no statistically significant difference in outcomes between the two arms.26 The median PFS and OS were 6.6 months (95% CI: 3.4–7.7) and 10.6 months (95% CI: 6.4–24.5), respectively, with chemoimmunotherapy compared with 3.9 (95% CI: 2.3–4.5) and 8.2 months (95% CI: 5.8–16.9), respectively, with dual ICIs.26 A large single–centre, phase II trial conducted in Asia, enrolled 128 patients to receive durvalumab or combination of durvalumb plus tremelimumab in addition to gemcitabine plus cisplatin.23 Initially, patients received immunotherapy following the first cycle of chemotherapy but after protocol amendment, all patients received immunotherapy concurrent with the first cycle of chemotherapy. Impressive ORRs were observed, with 72% in the chemotherapy plus durvalumab arm and 70% in the chemotherapy plus durvalumab and tremelimumab arm. The most common adverse events were cytopenias, with no unexpected safety events. The impressive results of this study formed the basis for development of the TOPAZ-1 trial.
TOPAZ-1 trial
The TOPAZ-1 trial evaluated the addition of durvalumab to the standard chemotherapy regimen of gemcitabine and cisplatin as a first–line systemic treatment option for patients with advanced or recurrent BTCs.25,29 TOPAZ-1 was a phase III, double-blind, randomized and placebo-controlled study in which patients were assigned to receive either durvalumab or placebo with gemcitabine and cisplatin. The primary outcome was OS and secondary outcomes included PFS, response rates and safety. Inclusion criteria required patients to have previously untreated BTC that was either unresectable or metastatic or who developed recurrence more than 6 months after curative intent surgery and completion of adjuvant therapy. Patients with ampullary carcinoma or those with exposure to prior immunotherapy were excluded. In the trial, patients could receive up to eight cycles of chemotherapy and if they had stable disease or better response, they were switched to maintenance durvalumab every 4 weeks or placebo, which they continued until progression or unacceptable toxicity.
A total of 685 patients were enrolled, with 341 receiving durvalumab with standard chemotherapy and 344 receiving placebo with standard chemotherapy. Baseline characteristics were similar in both groups. The majority of patients were Asian (56%) and had intrahepatic CCA subtype (56%). Only 1% of the patients had MSI-H tumours. The study met its primary endpoint, with median OS of 12.8 months (95% CI: 11.1–14.0) in the durvalumab group and 11.5 months (95% CI: 10.1–12.5) in the placebo group (p=0.021). OS rates at 12, 18 and 24 months were 54.1%, 35.1% and 24.9%%, respectively, compared with 48.0%, 25.6% and 10.4%, respectively, for placebo. Median PFS was 7.2 months (95% CI: 6.7–7.4) in patients who received durvalumab and 5.7 months (95% CI: 5.6–6.7) in those who received placebo (p=0.001). Multiple subgroup analyses for OS and PFS were conducted to examine the effect of features such as sex, age, PD-L1 expression, cancer subtype, race and Eastern Cooperative Oncology Group Performance Status. In all subgroup analyses, there was a trend favouring the durvalumab arm in both OS and PFS. When patients were stratified by PD-L1 positivity, there was no difference in outcomes between those who received durvalumab or placebo, suggesting that PD-L1 status may have limited value in predicting clinical benefit. Finally, ORR was 26.7% in the durvalumab arm and 18.7% in the placebo arm. Treatment–related adverse event rates were similar between the two groups. The incidences of grade 3 or 4 adverse events were 75.7% and 77.8% in the durvalumab and placebo arms, respectively. Only 6% of the patients discontinued durvalumab due to adverse events.
The updated results presented at the 2022 European Society for Medical Oncology congress, with an additional 6.5 months of follow–up, continued to demonstrate OS benefit with durvalumab plus chemotherapy compared with placebo plus chemotherapy (median OS 12.9 months [95% CI: 11.6–14.1] versus 11.3 months [95% CI: 10.1–12.5], respectively; hazard ratio 0.76).30 The 2–year OS rate was 40.6% among patients achieving response to treatment with chemoimmunotherapy compared with only 20.7% in patients who achieved stable disease. All patient subgroups continued to benefit in the updated analyses as well. Subgroup analysis by genomic alterations could not identify any particular alteration including KRAS, TP53, CDKN2A, ARID1A, IDH1 and FGFR that would predict response or lack of benefit to durvalumab treatment.
This study is the first phase III trial to include chemoimmunotherapy in the treatment of BTC. The addition of durvalumab to chemotherapy led to median OS improvement of 1.3 months. More importantly, there was an improvement in the 2–year OS rate from 10% to 25% with durvalumab, suggesting the possibility of more durable long–term survival in a minority of BTC patients represented in the tail of the survival curve. Given the above findings, the US FDA approved durvalumab in combination with gemcitabine and cisplatin for adult patients with locally advanced or metastatic biliary tract cancer.31
One of the major criticisms of the trial design was that patients received a maximum of eight cycles of chemotherapy followed by durvalumab or placebo as maintenance therapy until progression. This trial design was based on the ABC-02 trial, which established gemcitabine plus cisplatin as the standard of care. However, in typical clinical practice in the USA, treatment with gemcitabine plus cisplatin is continued until unacceptable toxicity or progression of disease, especially in patients responding to treatment. Cisplatin is sometimes difficult to continue beyond eight cycles due to nephrotoxicity and ototoxicity, but gemcitabine is typically continued until progression. The separation in survival curves between the two arms started at 6 months, coinciding with cessation of chemotherapy. Following the results of this trial, it remains unknown whether the current clinical practice should be changed or whether chemotherapy plus immunotherapy could be continued beyond eight cycles. This trial demonstrated an OS benefit of durvalumab in both Asian and Western populations, although the improvement was more pronounced in Asian population. Nonetheless, this was a global study including both Asian and Western populations, suggesting general applicability of trial findings.
The KEYNOTE-966 randomized clinical trial, comparing pembrolizumab with placebo in addition to gemcitabine plus cisplatin in first–line therapy for advanced, non-resectable CCA has finished accrual and is expected to read out in the near future.32 One key difference from the TOPAZ-1 trial is that gemcitabine can be continued until progression or unacceptable toxicity along with immunotherapy as part of maintenance therapy. The KEYNOTE-966 trial is expected to further address the question of whether immunotherapy adds benefit to chemotherapy as first–line treatment. Hopefully, this trial will also identify markers that could help in selecting patients who would derive maximum benefit from immunotherapy. It remains unknown whether the addition of anti-CTLA-4 would have any incremental benefit to chemotherapy plus anti-PD-1/PD-L1 blockade. In the previous phase II trial, the ORRs were similar when durvalumab or durvalumab plus tremelimumab were added to chemotherapy.23
Conclusions
Despite recent advances in the treatment of CCA, survival remains poor. Durvalumab in combination with gemcitabine and cisplatin chemotherapy should be considered as first–line standard–of–care therapy for patients with newly diagnosed locally advanced or metastatic CCA. However, the use of biomarkers including PD-L1 expression and TMB as a marker of response to immunotherapy in patients with CCA remains unclear. Several ongoing trials would further refine the role of immunotherapy in CCA (Table 228,32–42). FGFR2, NTRK, IDH, BRAF and HER2 alterations are now recognized as therapeutic targets. Further studies with biomarker–guided treatment strategies assessing novel therapeutic options are fundamental to improving survival in this population. The combination of immunotherapy and targeted therapy remains an active area of research.
Table 2: List of selected on–going clinical trials in biliary tract cancers utilizing immunotherapy
|
Trial |
Regimen |
Phase |
Line of therapy |
|
NCT0325027333 |
Nivolumab + Entinostat |
II |
2nd or later |
|
NCT0421116834 |
Toripalimab + lenvatinib |
II |
2nd or later |
|
NCT0429800835 |
AZD6738+ durvalumab |
II |
2nd or later |
|
NCT0505209936 |
FOLFOX + bevacizumab + atezolizumab |
Ib/II |
2nd or later |
|
NCT0545104337 |
Gemcitabine+ cisplatin + durvalumab+ tremelimumab + propranolol |
II |
1st |
|
NCT0494128738 |
Atezolizumab + varlilumab + cobimetiib |
I/II |
2nd or later |
|
NCT0532758239 |
Durvalumab + lenvatinib + nab-paclitaxel |
I/II |
2nd or later |
|
NCT0466092940 |
CT-0508+ pembrolizumab |
I |
2nd or later |
|
NCT0427814441 |
BDC-1001+ nivolumab |
I/II |
2nd or later |
|
NCT0523916942 |
Durvaluab + tremelimumab + capecitabine |
II |
Adjuvant |
|
NCT0400363632 |
Gemcitabine+ cisplatin + /- pembrolizumab |
III |
1st |
|
Sahai28 |
Nivolumab to 5-fluorouracil + liposomal-irinotecan |
I/II |
2nd |












