The term ‘head and neck cancer’ covers a large number of neoplasms with diverse natural history, most of which are uncommon and some of which are rare. These lesions, in over 85% of cases, represent squamous cell carcinomas of the mucosa of the upper respiratory and gastrointestinal tracts – head and necksquamous cell carcinoma (HNSCC) – and most are oral squamous cell carcinoma (OSCC). This review discusses HNSCC but focuses largely on OSCC; it does not include lip cancer.
Epidemiology
HNSCC is the sixth most common cancer worldwide. It affects males twice as commonly as females and shows a peak age at presentation between the fifth and seventh decades of life. In the US, HNSCC accounts for 2.8% of all malignancies. In Europe the disease shows its highest prevalence in Hungary, the north of France and Scotland. In India as well as in Brazil, OSCC accounts for 35% of all newly diagnosed cancers in men.
Aetiology
Lifestyle habits and demographic as well as genetic factors influence these geographical variations. The aetiology of HNSCC is well established in most instances – consumption of tobacco in any form and alcohol being the most common risk factors. The effects of lifestyle in the aetiopathogenesis of HNSCC include dietary habits, especially betel quid chewing and low fruit and vegetable consumption. Occasional cases are related to candidosis or syphilis, and exposure to the human papillomavirus (HPV) has been implicated as an aetiopathogenetic agent in oropharyngeal carcinoma, especially in young patients without exposure to tobacco or alcohol.
Diagnosis
HNSCC is usually diagnosed at a late stage, typically because of patient delay, and this has an adverse effect on prognosis. In the US, of the approximately 50,000 new cases of HNSCC diagnosed annually, over 60% are locoregionally advanced (stage III and IV) at diagnosis.
Potentially malignant disorders include:
- erythroplakia (red patch) – the most likely lesion to transform;
- leukoplakia (white patch); and
- lichen planus.
Far less common potentially malignant disorders include:
- discoid lupus erythematosus;
- dyskeratosis congenita;
- immunosuppressed states;
- oral submucous fibrosis;
- Paterson–Kelly syndrome (sideropenic dysphagia); and
- tertiary syphilis.
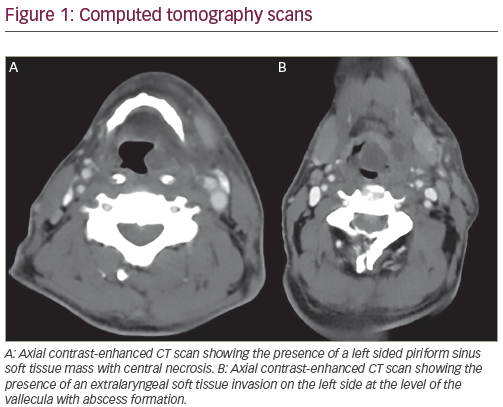
Any solitary oral lump, ulcer, white or red lesion persisting for more than three weeks, non-healing tooth extraction socket, numbness or unexplained loose tooth is suspect as oral cancer until proved otherwise (see Figure 1). Biopsy is mandatory. Second primary neoplasms are common: they are seen in up to 25% of patients with HNSCC over three years.
Management
The goal of treatment is to eradicate disease, preserve or restore form and function, minimise treatment sequelae and prevent any subsequent new primary cancers. Quality of life is crucial.
Factors that influence the choice of initial treatment regimen are related to the characteristics of the primary tumour, to the patient and to the therapeutic team. For the 40% of patients with HNSCC who present with early-stage disease, surgical resection or radiotherapy is recommended as a single treatment modality.
However, since most patients present with locally advanced disease (LAD), a multidisciplinary approach combining surgery, radiotherapy and chemotherapy is commonly indicated.1,2
Treatment combinations include:
- surgery followed by adjuvant chemoradiotherapy (CRT) or radiotherapy;
- CRT followed by surgery as a salvage treatment;
- induction chemotherapy followed by a definitive local therapy such as CRT; or
- targeted therapy with epidermal growth factor receptor (EGFR) inhibition combined with radiotherapy or CRT.
The patient’s acceptance of, compliance with and ability to tolerate a proposed therapeutic programme are crucial considerations in designing an optimal treatment strategy.
Factors related to the therapeutic team include experience, dexterity, ability and availability of technical support. Expertise in various disciplines including surgery, radiotherapy, chemotherapy, rehabilitation services, dental and prosthetic support, physiotherapy, speech therapy and psychosocial support are some of the areas crucial in bringing about optimal outcomes.
Tumour factors that affect the choice of initial treatment are site, size (T stage), location (anterior versus posterior), proximity to bone (mandible or maxilla) and histology (type, grade and depth of invasion) of the primary tumour, the status of cervical lymph nodes and previous treatment.
Early-stage Tumours
Outcomes of tumours treated by either surgery or radiotherapy are similar; therefore, treatment sequelae are an important consideration. Oncologists must also consider the disease site: oral cavity lesions are often treated with surgery followed by radiotherapy or CRT, whereas tumours in the oropharynx, hypopharynx, nasopharynx or larynx are usually treated first with CRT.3
Chemotherapeutic agents used to treat patients with HNSCC include cisplatin, carboplatin, taxanes, methotrexate, bleomycin and 5-fluorouracil, used either as monotherapy or in combination with radiation.
Advanced-stage Tumours
In patients with advanced-stage disease, in many centres in the US, the UK and Canada the current treatment preference is surgical resection with immediate reconstruction followed by post-operative radiation therapy or post-operative concurrent chemo-radiotherapy. However, in central Europe, and especially in the countries that participate in the European Organisation for Research and Treatment of Cancer (EORTC) and the German, Austrian and Swiss cooperative group on tumours of the maxillofacial region (DOSAK) groups, preoperative chemo-radiotherapy is the standard treatment.
Outcomes
Treatment for HNSCC has permanent effects on organs essential for normal activities such as breathing, speaking, eating and drinking. Loss of weight and malnutrition due to both cachexia and an inability to take a normal diet are frequent. It is also common for HNSCC patients to have underlying health problems from heavy smoking and alcohol consumption. A large percentage of patients also suffer from diabetes and respiratory and cardiovascular diseases. The resulting co-morbidities can influence treatment options and complicate post-operative management. HNSCC patients facing therapies of all kinds require expert support before, during and after their treatment. Many need rehabilitation over a sustained period and, despite the best care, many patients still experience long-term problems.
In most HNSCCs, early stage at presentation permits a positive outlook, and outcomes are frequently good: patients with stage I and II disease have a five-year survival rate exceeding 85%. However, stage III and IV disease give a five-year survival rate between 20 and 30% regardless of the combination of treatment strategies. The role of surgery as a prime therapeutic modality must be redefined since it has proved unable to significantly improve survival of patients with HNSCC.
Recent Advances
Chemotherapy
Chemotherapeutic agents are increasingly used to enhance the effects of radiotherapy (chemo-radiation), but chemotherapy alone is rarely appropriate for HNSCC. Concomitant chemo-radiation therapy (CCRT) has an improved therapeutic effect for patients with unresectable locoregionally advanced HNSCC, with a distinct survival advantage over either radiotherapy or chemotherapy alone. Prolongation of survival is especially possible with multi-agent chemotherapy protocols with the use of targeted therapies given concomitantly with standard fractionation or hyper-fractionated radiotherapy. Survival rates of 30–60% at three and five years are now being reported.
However, the improved overall clinical outcome with CCRT using hyper-fractionated radiotherapy comes at the cost of significant treatment-induced morbidity, particularly mucositis, dermatitis, diarrhoea, cardiotoxicity and myelosuppression. Patient selection for these intense treatment regimens must take careful consideration of the patient’s overall clinical status.4 Treatment by experienced physicians with multidisciplinary radiotherapy, medical, surgical, nursing and other supportive care is needed to sustain these patients through such intense treatment protocols.5
By contrast, with concomitant targeted agents and radiotherapy combinations, treatment-related toxicity is lower, especially for patients who are ineligible for surgery, have low surgical curability rates or harbour tumours for which non-surgical therapy represents a more acceptable treatment. Definitive platinum-based CRT is an approach favoured for medically fit patients with unresectable LAD.6 Nevertheless, combining chemotherapy with radiotherapy brings with it the additional adverse effects of chemotherapy and an exacerbation of radiotherapy-associated toxicities.7 Significant progress has also been made in the integration of chemotherapy in treatment programmes for locally advanced HNSCC in patients who are not surgical candidates, and in whom RT, as the cornerstone modality for local control, plays a major role in achieving complete disease eradication.8–10 Although methotrexate is still widely used for palliation, platinum compounds are the most active agents for the treatment of HNSCC, but the median survival time still rarely exceeds six months.11 The median survival time falls to just over three months in patients who receive further treatment for disease failing platinum-based therapies.12
Targeted Therapies
Therapies to specifically target cell signalling pathways dysregulated in tumours have focused mainly on epidermal growth factor receptor (EGFR or HER1) inhibitors, particularly cetuximab. EGFR are expressed in most HNSCCs and overexpression is often associated with poor clinical prognosis, including reduced disease-free and overall survival.13–15
A landmark randomised phase III trial by Bonner et al. established the efficacy of combining cetuximab with RT in the treatment of LAD.16 In this study, cetuximab (400mg/m2 initial dose followed by subsequent weekly doses of 250mg/m2) was added to once-daily (70Gy/35 fractions), twice daily (72–76.8Gy/60–64 fractions) or concomitant boost (72Gy/42 fractions) radiotherapy in 424 patients with carcinomas of the oropharynx, hypopharynx or larynx suitable for definitive radiotherapy. The addition of cetuximab to radiotherapy led to a 32% reduction in the risk of locoregional progression (hazard ratio [HR] for locoregional progression or death was 0.68; p=0.05). The median duration of locoregional control was increased by nearly 10 months (from 14.9 months with radiotherapy alone to 24.4 months with cetuximab plus radiotherapy) and the three-year locoregional control rate was 47% compared with 34% (p<0.01). The combination of cetuximab and radiotherapy also led to a significant 26% reduction in the risk of death (HR 0.74; p=0.03) and an almost 20-month increase in the median overall survival time (49 versus 29.3 months; p=0.03). Treatment with the combination of cetuximab and RT was generally well tolerated. The incidence of grade 3 or greater adverse effects was similar in the two groups, with the expected exception of acne-like rash and infusion-related reactions. Cetuximab did not exacerbate the common effects associated with radiotherapy in this setting, such as mucositis, xerostomia, dysphagia, pain, weight loss and performance status deterioration.16 A quality of life analysis of these data demonstrated that the benefits of adding cetuximab to radiotherapy were achieved without any detrimental effects on quality of life.17 Over time, and along with the improved survival and locoregional control achievable with the aforementioned schedules of CCRT delivery, a shift in the patterns of treatment failure has been observed.18
Historically, locoregional disease control represented the most important concern in LAD management, in that the occurrence of distant metastases (DMs) was relatively less common. However, increasingly sophisticated delivery of CCRT has led to locoregional control (LRC) rates in excess of 90% and the emergence of DMs as the most frequent cause of treatment failure.8,19 This observation suggested a possible role for adding further elements of systemic chemotherapy to the entire treatment ‘package’ with the aim of improving global treatment success measures by decreasing this incidence of distal recurrence.20,21 In view of the above matters, significant interest in adding induction chemotherapy (ICT) to definitive non-surgical local therapies has re-emerged.22,23
The pivotal TAX323 and TAX324 studies have established taxanes, cisplatin and 5-fluorouracil (TPF) as the most effective ICT combination regimen, if the latter is chosen as the basis of a multidisciplinary programme for the treatment of LAD.24 Future directions for docetaxel-based therapies include combinations with other chemotherapeutic agents or novel biologic therapies – most notably agents targeted against the EGFR – and modification of CCRT.
As an increasing number of targeted therapies become available, we can hope to see more active combinations in the treatment of HNSCC. Indeed, there may be added benefit from using such combinations as part of ICT regimens, integrated into the radiotherapy part of the sequential therapy regimen or even as maintenance after the successful delivery of the local/regional component of the regimen (in complete responders).25 There is currently a paucity of data on molecular end-points that could be of prognostic and/or predictive value relevant to the use of TPF. Such data could help determine which patients (according to disease site, stage, HPV, tissue typing status, etc.) would most benefit from ICT.26
Technological advances have also facilitated the identification of several tumour-associated antigens now under investigation as therapeutic targets in HNSCC, including tumour-specific antigens and tumour-specific mutated proteins unique to the tumour and that may contribute to the malignant phenotype, e.g. tumour suppressor gene p53. Antigens overexpressed in tumours are a further category of targets under investigation. Notable examples are carcinoembryonic antigen (CEA), vascular endothelial growth factor (VEGF) and EGFR. Antigens derived from oncogenic viruses, such as the HPV E6 and E7 oncoproteins, are also potential targets in HNSCC.27,28
Conclusion
Targeted agents, particularly the EGFR inhibitors and the VEGF inhibitor bevacizumab, have added a new dimension to potential treatment. The recently reported results of the phase III Cetuximab (Erbitux) in Combination With Cisplatin or Carboplatin and 5-Fluorouracil in the First Line Treatment of Subjects With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (EXTREME) trial,29 demonstrating that the addition of cetuximab to standard chemotherapy prolongs overall survival in the first-line treatment of recurrent and/or metastatic HNSCC, perhaps represents one of the most significant clinical advances in the treatment of HNSCC during the last few decades. There is little doubt that biological treatments, along with the use of more sophisticated radiotherapy, will continue to evolve in the years to come.30