Multiple myeloma (MM) remains largely an incurable disease with only a small percentage of patients achieving long-term remission.1 Here, we highlight some of the major studies on MM presented at the American Society of Hematology meeting in December 2021.
Monoclonal gammopathy of undetermined significance and smouldering myeloma
The Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study is the largest population-based screening (n=75,422) and randomized study in which participants were assigned to different follow-up and monitoring strategies. Monoclonal gammopathy of undetermined significance (MGUS) was reported in 4.9% of study participants.2 The prevalence of MGUS increased with age with 2.3%, 6.2% and 12.9% of cases diagnosed in the age groups 40–59, 60–79 and 80–103 years, respectively. The prevalence of MGUS was higher in males, 5.9% versus 4.1% (p<0.0001), and most individuals had either low-risk (38%) or low-intermediate (36%) risk MGUS, followed by high–intermediate (26%) risk MGUS. High-risk MGUS accounted for 0.2% of all cases. In the intensive screening arm of this study where bone marrow examination and full staging were performed, 0.5% of study participants >40 years of age had smouldering multiple myeloma (SMM).3 While 36% of participants had intermediate to severe SMM according to the Mayo Clinic 20/2/20 risk stratification, close to 50% of participants were in this category per the PETHEMA criteria.4 Unsurprisingly, intensive diagnostic and monitoring strategies were able to identify more study participants with disease progression to SMM and full-blown MM.
The PROMISE study is the first of its kind in the USA that screened high-risk populations, namely African Americans, and/or first-degree relatives of patients diagnosed with haematological malignancies (leukaemia, lymphoma and Waldenström macroglobulinaemia) or precursor conditions to MM.5 Subjects with two or more family members with haematological malignancies were also included in the study. An impressive 7,622 subjects were screened using conventional serum protein electrophoresis, light chain assays and novel highly sensitive matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay. In this study, around 6% of the study population over the age of 50 have MGUS identified by conventional testing and 13–17% using the more sensitive, mass-spectrometry-based assay. These were higher than the 3% incidence of MGUS that was previously known, but close to the 5% reported by the iStopMM study.2,6 In contrast to the iStopMM study, where participants were a homogenous Icelandic population, the PROMISE study was enriched for a high-risk population as detailed above. Intuitively, the more sensitive mass spectrometry assay detected M protein that was lower than the limit of detection of conventional testing (<0.2 g/dL). This was referred to as monoclonal gammopathies of indeterminate potential (MGIP). Importantly, MGIP was detected in 28% of study participants over the age of 50 and indicated a worse overall survival (OS) due to all-cause mortality, much akin to clonal haematopoiesis of indeterminate potential. Interestingly, the prevalence of MGIP was similar across the high-risk group as defined by the PROMISE study and the control arm of non-high-risk participants.7 The clinical significance of MGIP needs to be further investigated. Overall, this study highlighted the higher incidence of MGUS, particularly in a high-risk patient population.
Existing risk stratification for SMM requires invasive bone marrow studies.4 A non-invasive peripheral blood next generation flow cytometry assay was able to detect circulating tumour cells (CTC) in 78% of patients with SMM.8 In a cohort of untreated SMM patients, CTC levels of ≥0.02% indicated a high risk of progression to MM compared with those with <0.02% or undetectable CTC (time to progression [TTP] 11 months versus not reported; p<0.001). CTC of <0.02% distinguished low-risk from intermediate- or high-risk SMM groups, but not intermediate-risk from high-risk SMM groups as defined by the Mayo 20/20/2 risk stratification.4 CTC level was an independent prognostic factor for TTP along with M protein and serum free light chain, while percentage of bone marrow tumour cells was not significant in this study. The use of non-invasive peripheral blood-based CTC detection using a next-generation flow cytometry assay could allow frequent monitoring and detection of an evolving pattern of disease progression in this subset of patients, especially with the field moving towards identifying and treating
high-risk SMM.4
Preliminary results of studies investigating early treatment in high-risk SMM and high-risk MGUS9 were also reported. A phase II study of an intensive MM-like treatment approach with high-dose chemotherapy and autologous stem cell transplant (ASCT) in high-risk SMM reported a 94% progression free survival (PFS) at 55 months of follow-up.10 The phase II study of the combination of ixazomib, lenalidomide and dexamethasone (IRd) in high-risk SMM demonstrated an overall response rate (ORR) of >90% and deep remission (very good partial responses [VGPR] and complete responses [CR]) in >40% of study participants.11 Overall, the current research focusing on precursor states of MM and long-term follow-up will be important to determine if early detection and treatment would improve the quality of life and, importantly, the OS of patients.
Frontline therapies in newly diagnosed multiple myeloma
Summaries of some of the major phase II and III studies in newly diagnosed MM (NDMM) are presented in Table 1.
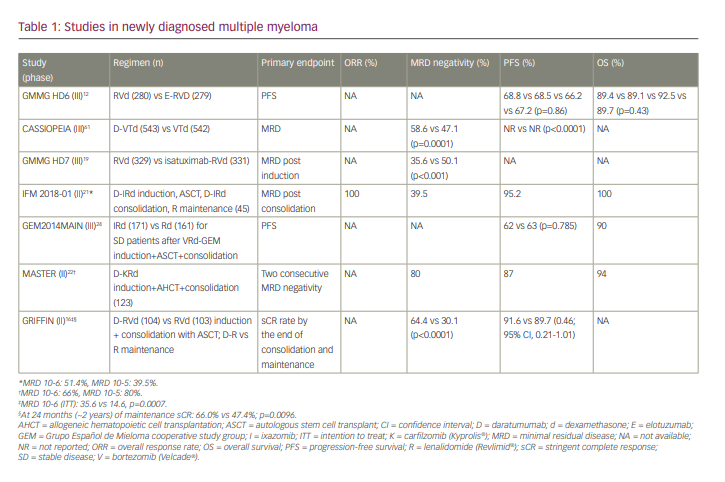
The phase III German-speaking Myeloma Multicenter Group (GMMG HD6) trial studied the addition of the anti-SLAMF7 monoclonal antibody (mAb) elotuzumab to lenalidomide, bortezomib and dexamethasone (RVd) in induction, consolidation and maintenance treatment in transplant-eligible NDMM.12 Along the lines of the ELOQUENT-113,14 and SWOG 121115 studies, the addition of elotuzumab did not result in improved PFS or OS in NDMM. Is this the end of the road for elotuzumab therapy in NDMM?
In contrast, the addition of the CD38-targeting mAb (CD38 mAb) daratumumab and isatuximab to the RVd backbone in NDMM has been encouraging. In an updated analysis of the phase II GRIFFIN study,16 the addition of daratumumab to RVd induction and consolidation in conjunction with ASCT, followed by daratumumab plus lenalidomide maintenance, continued to demonstrate deep and durable responses in transplant-eligible NDMM. At a median follow-up of 27.4 months, the study arm had a higher rate of stringent CR (sCR) (66.0% versus 47.4%; p=0.0096) and bone marrow minimal residual disease (MRD) negativity17 (10-5 threshold: 64.4% versus 30.1%, p<0.0001; 10-6 threshold: 35.6% versus 14.6%; p=0.0007) compared with the control arm.18 Although the study was not powered to report a difference in PFS, there was a positive trend towards an improvement in PFS with the addition of daratumumab to RVd.
The GMMG HD7 study is a large phase III randomized controlled trial investigating the quadruple regimen of isatuximab-RVd in transplant-eligible NDMM. Unlike the previous studies of quadruple therapy in MM, this study was uniquely powered to compare the rates of bone marrow MRD negativity (at 10-5) at the end of induction therapy. The study met its primary endpoint of higher rates of bone marrow MRD negativity at the end of the 18-week induction treatment with isatuximab-RVd (isatuximab-RVd 50.1% versus RVd 35.6%). Importantly, the addition of isatuximab was well tolerated with no major side effects.19 Both GRIFFIN and GMMG HD7 continue to support the enhanced efficacy with deeper responses including higher rates of MRD negativity of quadruplet induction over triplet induction in NDMM patients; however, it remains to be proven if these will improve the OS.
The triplet induction and consolidation regimen of IRd is a safe, convenient and effective regimen in transplant-eligible NDMM patients, as has been shown by the Intergroupe Francophone Du Myélome (IFM) study.20 The current phase II IFM 2018-01 study builds on this and examines daratumumab added to IRd as extended induction prior to, and as consolidation following ASCT in standard-risk NDMM younger than 66 years.21 The primary endpoint was MRD negativity after consolidation and before maintenance. MRD testing was performed using next-generation sequencing with a sensitivity threshold of detection of MM cells up to 10-6. Around 39.5% of patients were MRD negative after consolidation, with 2-year PFS at 95.2% and 2-year OS at 100%. While ixazomib is an oral proteasome inhibitor with a favourable side-effect profile and negligible neurological or cardiovascular toxicity, the reported MRD negativity rates were lower than those obtained with the combination of CD38 mAbs with bortezomib.18
In the updated analysis of the MASTER study,22 a daratumumab-based quadruplet therapy, allogeneic haematopoietic cell transplantation and bone marrow MRD response-adapted consolidation therapy led to high rates of MRD negativity (80%) in NDMM. A similar proportion of patients with standard-risk, one, and ≥2 high-risk cytogenetics reached overall MRD negativity (78% versus 82% versus 79%, respectively) and MRD negativity at a sensitivity of <10-6 (64% versus 73% versus 58%, respectively). Two-year PFS and OS rates of 58% and 75%, respectively, were reported for patients with ≥2 high-risk cytogenetics in contrast to the 2-year PFS of 91% and 97% and 2-year OS of 96% and 100% in subjects with standard-risk and one high-risk cytogenetic, respectively. Thus, treatment-free periods in patients with standard-risk disease and one high-risk cytogenetic did not significantly increase the risk of progression or bone marrow MRD resurgence (International Myeloma Working Group [IMWG] criteria). This study showed that an MRD status-adapted treatment could be an option, particularly in standard-risk MM patients, and more importantly emphasizes the need for novel approaches in the subset of patients with ≥2 high-risk cytogenetics.
The GEM2014MAIN trial studied ixazomib in the maintenance setting post ASCT. Patients with at least stable disease23 after the GEM2012menos65 trial regimen of bortezomib, lenalidomide and dexamethasone were randomized to receive maintenance treatment with IRd versus lenalidomide and dexamethasone (Rd).24 The study attempted an MRD-risk–adapted maintenance strategy whereby patients with bone marrow MRD negativity after 2 years of maintenance treatment were able to be off therapy, while patients with MRD persistence were to continue Rd for an another 3 years. After a median follow-up of 56 months, there was no difference in PFS between the two maintenance arms (PFS at 5 years: 62% versus 63% with IRd and Rd, respectively, p=0.785). The study used dexamethasone alongside ixazomib and side effects such as thrombocytopenia and gastrointestinal upset were higher with ixazomib. Ixazomib dose modification was required in 31% and discontinuation in 9% of patients and this could have impacted the lack of improvement in PFS noted in this study. Previously, TOURMALINE MM325 and MM426 reported an improvement in PFS with ixazomib maintenance therapy compared with placebo in transplanted and non-transplanted MM patients, respectively. Head-to-head comparison of ixazomib with the current standard of care maintenance regimen of lenalidomide has not been formally conducted and the current role of ixazomib in the maintenance setting in MM is unclear.
There were several exciting studies on the management of transplant-ineligible NDMM patients. The HOVON 143 is a prospective, multicentre, single-arm, open-label phase II trial of transplant-ineligible, intermediate-fit NDMM patients. Results presented were following the induction regimen of nine 28-day cycles of combination daratumumab, ixazomib and dexamethasone. The ORR was 71% with 35% attaining VGPR and 2% CR. The median PFS was 17.4 months. While the study showed this triplet induction was feasible and effective, around 46% of patients did not proceed to the maintenance regimen of ixazomib and daratumumab. Of note, treatment discontinuation due to toxicity (6%) and noncompliance (5%) remains a concern.27 There is a pressing need to optimize treatment regimens while minimizing adverse effects without compromising efficacy.
FiTNEss is a phase III multicentre randomized controlled trial that recruits transplant-ineligible NDMM patients with different levels of frailty and has no performance-status-based inclusion and exclusion criteria. Patients are randomized to a standard dosing (dose modifications to toxicities) arm and study arm where frailty index (based on IMWG score)-adjusted induction therapy will be tested.28 Patients will receive an all-oral regimen of IRd. The preliminary results of the study presented at ASH 2021 demonstrate the feasibility of recruiting older, less fit patients to clinical trials.29
Therapies in relapsed refractory multiple myeloma
Triple-class refractory (TCR) MM, defined as refractory to proteasome inhibitors, immunomodulatory drugs (IMiDs) and CD38 mAbs, is an emerging major therapeutic challenge.30 Since the approval of CD38 mAbs in NDMM, TCR MM is increasingly encountered at an early stage of the disease.31 There is an unmet need for novel approaches in this group of patients. Some of the major studies in the relapsed/refractory MM (RRMM) space are highlighted below.
The B-cell lymphoma 2 (BCL2) inhibitor venetoclax (Ven) combined with bortezomib and dexamethasone (Vd) showed promising efficacy, particularly in MM with t(11;14).32 The BELLINI trial33 is a randomized, double-blind, multicentre phase III study of Ven or placebo combined with Vd in RRMM. Addition of Ven to Vd prolonged PFS compared with placebo (hazard ratio [HR] 0.58; 95% confidence interval [CI] 0.43–0.78; p=0.0003) with foremost benefit seen in patients with t(11;14) or high BCL2 expression (HR 0.12; 95% CI 0.03–0.44). In the final OS analysis, an increased mortality signal was noted in the study arm primarily driven by early treatment-emergent death (HR 1.19; 95% CI 0.80–1.77). There was no difference in OS in the subgroup with t(11;14) or high BCL2 expression (HR 0.82; 95% CI 0.40–1.70; p=non significant). The researchers concluded that VenVd has a favourable benefit–risk profile in MM with t(11:14) or high expressers of BCL2, particularly if adequate supportive care such as infectious disease prophylaxis could be reinforced. Preliminary reports of a phase I/II study investigating Ven (at doses of 400 mg or 800 mg), daratumumab and dexamethasone (Dd) versus daratumumab, bortezomib and dexamethasone (DVd) in RRMM with t(11;14) were promising. The preliminary ORR was 72.7%, 100% and 62.5% for the Ven 400mg Dd, Ven 800 mg Dd and DVd arms, respectively, with no major safety concerns.34 These results mark a new era of biomarker-driven therapy in MM.
Frail relapsed MM patients have inferior outcomes due to high treatment discontinuation rates secondary to adverse effects. In the phase II IFM 2018-02 study, ixazomib daratumumab (I-Dara) without dexamethasone was investigated in elderly frail patients with RRMM. The median age was 86 years with the majority of patients enrolling at first relapse. All patients had prior exposure to bortezomib and had an IMWG fragility score of ≥2. In this study, I-Dara yielded an ORR of 86% with ≥VGPR rates of 33% in patients with ongoing treatment. Around 54% of patients suffered from grade ≥3 adverse effects and half of these were serious adverse effects highlighting the unmet need for treatment with a better safety profile in this patient population.35
Belantamab mafodotin, a novel anti-B-cell maturation antigen (BCMA) antibody drug conjugate, displayed single-agent activity in the phase I DREAMM-1 and phase II DREAMM-2 studies in heavily pretreated patients with RRMM. Despite US Food and Drug Administration (FDA) approval in the TCR patient population, the toxicity profile of corneal keratopathy has resulted in serious treatment delays and cessations, potentially limiting the clinical efficacy.36,37 Single centre, real-life experience with belantamab mafodotin showed that 74% of patients developed keratopathy at a median of 1.4 months from starting therapy. Around 38% of patients developed keratopathy after one cycle of treatment and the majority were grade 1 (26%) and grade 2 (29%). ORR was 32%, consistent with results of the DREAMM-2 study;38 however, in 73% of patients, therapy doses were interrupted due to toxicity with resultant disease progression in half of these patients.39 Overall, in 15% of patients in this cohort of 34 patients, the drug was permanently discontinued due to keratopathy. The current Risk Evaluation and Mitigation Strategy programme requires baseline and follow-up eye examination at every cycle and this could be resource intensive and time consuming for patients. To overcome this, other approaches such as utilizing patient-reported ocular symptoms to guide dose modification are also being studied.40 Several ongoing trials are investigating alternative treatment dosing and frequency schedules to optimize the risk–benefit profile.41,42
Iberdomide, an oral cereblon E3 ligase modulator, has shown clinical efficacy in RRMM. The phase I/II dose-expansion/escalation study enrolled a heavily pretreated patient population with a median of six prior lines of therapy and 97% of study subjects were TCR. Around 25–30% of the patients also had extramedullary disease, a marker of higher-risk disease. Iberdomide as a single agent showed an ORR of 26.5% with a favourable tolerability profile.43 Iberdomide is also being examined in combination with other agents in RRMM.44
Major bispecific antibody and chimeric antigen receptor (CAR) therapy clinical trials have been summarized in Table 2.
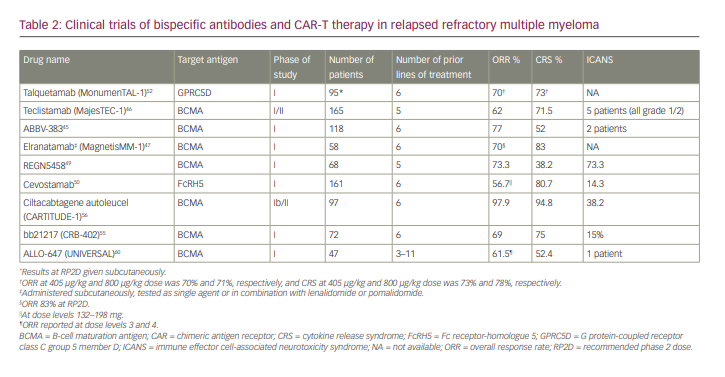
Bispecific antibodies targeting novel targets such as BCMA, G protein-coupled receptor class C group 5 member D (GPRC5D) and Fc receptor-homologue 5 (FcRH5) are being investigated in the RRMM space.
ABBV-383 (formerly TNB-383B) is a BCMA x CD3 T-cell–engaging bispecific antibody. In an update of the phase I dose-expansion study, an ORR of 77% was observed at doses ≥40 mg. Cytokine release syndrome (CRS) was seen in around 52% of patients, mostly grades 1 and 2.45
Teclistamab is another BCMA x CD3 bispecific antibody engineered to enhance the immune response against tumour cells by binding to CD3 on T-cells and BCMA on plasma cells, thus promoting T-cell–mediated killing of BCMA-positive myeloma cells.46 The phase I/II MajesTEC-1 is a first-in-human, dose-escalation/dose-expansion trial of teclistamab in RRMM. The current analysis reported an ORR of 62% in triple-class exposed RRMM. Teclistamab was well tolerated with a manageable safety profile and ongoing trials are examining teclistamab in earlier lines of treatment and in combination with other therapy.
Elranatamab (PF-06863135) is a humanized bispecific antibody targeting BCMA. MagnetisMM-1, a phase I study of elranatamab either as single agent or in combination with lenalidomide and pomalidomide, reported an ORR of 70% across the efficacious dose range with CR/sCR in 30% of study participants. Interestingly, 22% of patients had prior BCMA-targeted therapy and among these, 75% (three out of four patients) response rates were reported. The drug also has a manageable safety profile with low-grade CRS occurring in the majority of patients (83%).47 MagnetisMM-3, the phase II study, will test elranatamab in TCR MM and will enrol patients into two independent parallel cohorts: BCMA-naïve and previous exposure to BCMA.48
In the updated analysis of the phase I study of REGN5458, a BCMA-targeting bispecific antibody, an ORR of 73.3% was noted in RRMM patient population. Overall, CRS rates were lower at 38.2% with grade 2 CRS reported in only 4.4% of patients. There was also no grade 3 CRS or neurotoxicity reported with this agent.49
Cevostamab is a novel, humanized T-cell–engaging bispecific immunoglobulin (Ig) G antibody which targets CD3 on T-cells and FcRH5 on MM cells. In the updated results of the phase I dose-escalation and dose-expansion study of cevostamab in RRMM, an ORR of 56.7% was reported, with doses ranging between 132–198 mg. There is an unmet need for therapeutics targeting non-BCMA markers and further clinical development is underway.50
Talquetamab is a bispecific IgG4 antibody that binds to GPRC5D on MM cells and CD3 receptors on CD3+ cells to induce T-cell–mediated cancer cell death.51 Reports from the ongoing phase I MonumenTAL-1 trial reported the safety and efficacy at the recommended phase II doses of 405 µg/kg (weekly) and 800 µg/kg (biweekly) in RRMM. In the current analysis, an ORR of 70–71% was reported across the two different dose ranges. Apart from the CRS and immune effector cell-associated neurotoxicity syndrome (ICANS), which are expected of this class of T-cell–directed therapy, the unique side effects included skin-related adverse effects noted in 65% of patients. Preliminary results of the TRIMM-2 study of talquetamab with daratumumab in RRMM patients were also reported. Overall, the combination was well tolerated with safety comparable with monotherapy.52 Talquetamab is being studied as monotherapy and in combination with other agents in RRMM.
In general, the bispecific monoclonal agents are highly active in RRMM with ORR ranging between 60–97%. Generally, CRS was early onset and manageable with timely intervention and step-up dosing has further attenuated its risk. There are fewer cases of ICANS with this class of drugs. This favourable side-effect profile will enable ease of administration, particularly in the outpatient and community healthcare settings in the future. The robust depletion of plasma cells with BCMA-targeting bispecific antibodies can result in profound and prolonged hypogammaglobinaemia and risk of infectious complications as noted with this class of drugs.53 Unlike CAR-T therapy, which is a one-time treatment, bispecific antibodies are currently administered as continuous therapy until disease progression.
Idecabtagene vicleucel (ide-cel, bb2121) is the first CAR-T cell therapy to secure FDA approval for the treatment of relapsed or refractory MM following four or more prior lines of therapy. Currently, many innovative CAR-T therapies are in the development pipeline.
bb21217 is a BCMA-directed CAR-T cell therapy which uses the same CAR molecule as ide-cel, with the addition of a PI3K inhibitor during ex vivo culture to enrich for memory-like T cells and decrease the proportion of highly differentiated or senescent T cells. Enrichment for memory-like CAR-T cells could increase the CAR-T persistence and positively influence duration of response. Accordingly, the CAR-T cell persistence was greater (81% and 60% at 6 and 12 months, respectively) compared with ide-cel or bb2121 ( 59% and 36% at 6 and 12 months, respectively).54 The duration of response was 17.0 months (compared with 10.7 months overall and 11.3 months at the 450×106 dose with ide-cel or bb2121),54 but a larger proportion of patients in this study received the higher dose of 450×106 CAR-T cell. In the 72 patients enrolled in this phase I study, the ORR at higher doses was 74% with a CR rate of 28%. CRS and ICANS rates were 75% and 15%, respectively.55 Unfortunately, in a recent update further development of this CAR-T product has been permanently halted.
Ciltacabtagene autoleucel (cilta-cel) is an investigational autologous CAR-T cell therapy comprising two BCMA-targeting single-domain antibodies intended to boost avidity and improve clinical efficacy. Cilta-cel showed early, deep and durable responses in the phase Ib/II CARTITUDE-1 study in heavily pretreated patients with RRMM. Current analysis reports updated results from CARTITUDE-156 with longer duration follow-up (median: ~2 years). The ORR was 97.9% with stringent CR rates of 82.5% and VGPR rates of 94.5%. Two-year PFS and OS were 60.5% and 74.0%, respectively. In addition to CRS and ICANS, unique delayed neurotoxicity was reported in 12% of patients with 9% of these being grade 3 and 4.57 There were no new safety signals including new neurotoxicity or neurocognitive events observed with longer follow-up. Ongoing trials incorporate cita-cel in earlier lines of therapy (CARTITUDE 2, CARTITUDE 4) and in NDMM patients (CARTITUDE 5).
BCMA is shed from tumour cells through enzymatic cleavage by the gamma secretase complex and tumour cells with low levels of BCMA could thus escape recognition by CAR-T cells. The phase I first-in-human trial of escalating doses of BCMA CAR-T cells in combination with a gamma secretase inhibitor (GSI, JSMD194) enrolled 18 patients with RRMM.58 A significant proportion had prior treatment with BCMA-targeted therapy (39%) and CAR-T therapy (22%). The ORR was 89% with CR rates of 44%. Of note, CRS rates were higher at 94% with grade ≥3 noted in 28% of cases. ICANS data were not reported. The combination of BCMA CAR-T and GSI could augment anti-tumour activity, even when very low doses of BCMA CAR-T cells are administered.
The phase I first-in-class trial of GPRC5D-targeting CAR-T cell therapy, MCARH109, demonstrated a promising safety profile in RRMM. This study enrolled heavily pretreated RRMM patients with a median of eight prior lines of therapy. Of the 12 patients treated, 58% had received prior BCMA- targeting therapy including 50% who received prior BCMA CAR-T therapy. This CAR-T construct was able to induce high rates of clinical response with a very favourable side-effect profile of very low rates of high-grade CRS and neurotoxicity.59
Allogeneic CAR-T therapy addresses inherent issues with autologous CAR-T which includes delay in manufacturing and variable product quality due to poor T-cell yield. ALLO-715 is the first of its kind, genetically modified anti-BCMA CAR-T with a 4-1BB costimulatory domain.60 The construct has two key attributes to mitigate the risk of graft-versus-host disease (GvHD); namely knock-out of CD52 which allows use of ALLO-647, an anti-CD52 mAb, during lymphodepletion without affecting CAR-T cells, and disruption of the T-cell receptor alpha constant gene. UNIVERSAL is an open-label, phase I trial in RRMM with ≥3 prior lines of therapy including a proteasome inhibitor, IMiD and CD38 mAb. ALLO-715 was well tolerated with low-grade CRS and reversible neurotoxicity. There was no GvHD reported in this study and the ORR (61.5%) was comparable to the autologous product.
BCMA CAR-T therapies have showcased impressive response in heavily pretreated RRMM. CAR-T therapy is an attractive one-time treatment that can induce deep and durable response. Several issues such as understanding ideal patient and disease characteristics, access to academic or transplant centre, outpatient administration and management of adverse effects and cost of therapy need further clarification. Taken together, the multitude of therapeutic options highlighted in this review have set a high benchmark for the management of RRMM.