An increasing proportion of elderly people attend medical oncology consultations in which many questions about appropriate treatment will be raised by the oncologist. The balance between treatment benefits (in terms of further life expectancy of the elderly) and risks (hematologic and nonhematologic toxicity, functional impairment, and even death) will be discussed. In many cases, the treatment of elderly cancer is suboptimal, especially because of concerns of inducing severe toxicity in this population. Age alone should not be used as the only factor in making decisions. We must also consider other factors, such as comorbidity, functional status of the patient, cognitive and mood status, nutritional status, and social risk. In order to evaluate each of these factors, it is crucial to perform a Comprehensive Geriatric Assessment (CGA) as well as a specific training in Oncogeriatrics, which should be mandatory for Oncologists. In this article, we will briefly describe the current situation in Oncogeriatrics, the peculiarities of the treatment of elderly patients, and which tools are valuable to comprehensively assess the elderly patient, with the intention of getting optimal treatment in terms of maximum efficacy and minimal toxicity.
Epidemiology of Cancer in the Elderly
In the US and Europe, the elderly account for 13–15% of the total population1 (old age is defined as 65 years or older). However, this broad definition cannot distinguish between patients in different stages of aging. Sometimes it is useful to consider three different stages: the old–young (the young–old), between 65 and 70 years; the elderly elderly (the old–old), between 75 and 84 years; and older-elderly (the oldest–old), i.e. 85 years or more. Currently, 50% of malignant tumors occur in people 65 years or older. The final outcome data on survival and epidemiology (SEER [Surveillance, Epidemiology and End Results]) of the National Cancer Institute suggests that people more than 64 years are 11 times more likely to develop cancer than people between 25 and 44 years and this risk is two to three times higher than in those between 45 and 64 years. As a result, more than two-thirds of patients with certain cancers (colon, rectum, stomach, pancreas, bladder) are 65 years old or more, and over 50% of all hematologic malignancies are diagnosed in the elderly.2,3 If current trends of population growth continue, in 2020, approximately 60% of all cancers will affect the elderly.4
The annual crude incidence of cancer in Europe is 338 per 100,000 inhabitants in Eastern Europe, and 447 per 100,000 population in Western Europe5 and this incidence increases with age.
Due to population growth, the management of elderly cancer patients represents a challenge for the medical community. Life expectancy has increased in both men and women. It is estimated that life expectancy can exceed 90 years in women in industrialized countries.6 Approximately 70% of deaths attributed to cancer occur in people 65 years or older. It is clear that 35% of cancer deaths in men and 46% of cancer deaths in women occur after age 74. Cancer-specific mortality is still increasing according to age, and despite considerable progress in the management and treatment of cancer in the general population, mortality in the elderly has increased by 15–20% between 1970 and 1994.7 In Europe, the 5-year survival adjusted for age, of any tumor, was 55.8 % (95 % confidence interval [CI] 55.3–56.2%). In the US it was 62.9% (95% CI 62.6–63.2 %) and in Spain, 59.0% (95 % CI 56.9–61.2%).8
But cancer also makes a dramatic impact on the autonomy of the elderly. Cancer increases the progressive deterioration that occurs during aging. In 2050, the dependency ratio will rise from 22% to 46%.9 Cancer is much higher in elderly than in young people. So, if there is not a good treatment in this population, there will be a negative impact on prognosis.10
Physiology of Aging
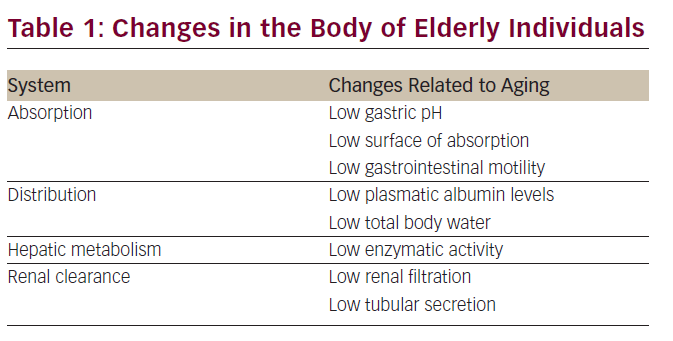
Chemotherapy is one of the most important weapons in the fight against cancer. Although many trials have clearly shown that chemotherapy is feasible in the elderly, doctors fear its side effects and chemotherapy use is limited in the elderly.11,12 It is usual to give suboptimal treatment in the elderly.13 Age decreases adaptability to external changes and the effectiveness of control mechanisms, which is reflected in slow responses, such as fluid balance control, blood glucose control, blood pressure control, etc. Moreover, with aging, body composition is modified, as it increases the plasma volume, decreases total body and extracellular water, and increases adipose tissue. With aging, a number of physiologic changes occur that are related to absorption, distribution, and hepatic metabolism of drugs. With this in mind, it is necessary to take particular care of medication in the elderly, and therefore, chemotherapy.14–16 Pharmacokinetic changes associated with aging as well as diseases that impact on the dosage of the drugs are shown in the following table (see Table 1).17,18
Pathophysiology of Frailty
Evidence suggests that elderly people have compensation mechanisms to maintain their functional reserve. For example, Frost interpreted that osteoporosis is an attempt by the body to reduce the weight of the bones when they have become too heavy to be moved by muscles already weakened.19
Compensation is good, but we must not forget that people who are compensating also have a high risk for decompensation. For example, if a person is using the reserve to offset muscle balance problems, muscle strength may not be available during other critical situations. Thus, based on the terms of ‘susceptibility and compensation,’ it is understandable why the elderly are cognitively ‘frail.’ Many times, although they are perfectly orientated in time and space when they are in their normal environment, they can become severely delirious after being hospitalized (cognitive frailty).20 The concept of brittleness and loss of reserves can be especially helpful to Oncologists. It is known that the elderly are more susceptible than younger people to myelosuppression secondary to chemotherapy. Myelotoxic effects are more prolonged and severe as elderly patients have a lower hematopoietic reserve, caused by aging or an age-related comorbidity.21 This decrease in the hematopoietic reserve is usually compensated and does not affect the basal granulocyte number, so it is not initially detected. However, the ‘frailty of the hematopoietic system’ has severe consequences to stressful situations (e.g. the administration of chemotherapy). All this highlights the importance of understanding tools to assess the physiologic reserve. Recent research in the Unit of Cancer in the Elderly in the Section of Medical Oncology at the General Hospital Virgen de la Luz de Cuenca, attempts to answer many of the questions.22
Comprehensive Geriatric Assessment in Medical Oncology
The CGA is a tool to analyze the health status of the elderly. The health status of this population depends on factors beyond medical problems, such as social and psychologic factors and mental state. The CGA also emphasizes the assessment of functional status, both for its preservation, and for its improvement. The CGA is a diagnostic process that is based on three pillars: a multidisciplinary, multidimensional assessment, a process of analysis and correlation of patient characteristics, and the creation of an individualized intervention plan. The CGA is based on the premise that the systematic evaluation of the elderly by a team of professionals can discover treatable health problems.23
The value of the CGA in oncology focuses on the achievement of different objectives that, in turn, are heterogeneous: to increase survival, to avoid functional decline, to prevent readmissions or hospital admissions for chronic health problems not recognized with a simple routine medical evaluation, to increase or decrease tolerance to cancer treatment toxicity, and to increase or maintain the quality of life of elderly patients. Currently, in Oncogeriatrics, the CGA is useful for: detecting reversible factors that interfere with treatment (inadequate social support, malnutrition, reversible comorbidity, etc.); estimating the risk for mortality according to the functional state of the patient; the degree of comorbidity (e.g. depression and anemia are associated with increased mortality) and the presence of geriatric syndromes; and estimating tolerance to chemotherapy, which is lower in patients with functional dependency, comorbidity, malnutrition, and/or anemia.24
There is no unique model of CGA in Oncogeriatrics. Different models have been published in the scientific literature, but each of them has been applied interchangeably to several types of cancer and tumoral stages.
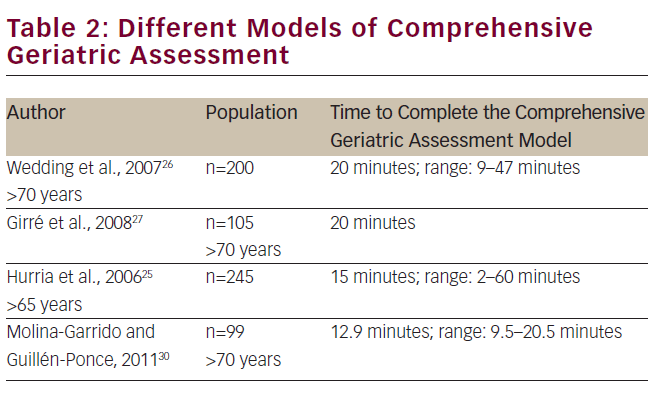
Table 2 lists some of these models, which have been the basis for further development of other models.25–27 Other well-known models of CGA have been proposed by Balducci and Ingram et al.28,29
In Spain, we developed a model for CGA that was composed of scales used and validated in the Spanish geriatric population. Although this model is not comparable with the rest of models used in other studies, in absolute values, the number of minutes necessary to apply the Spanish model of CGA is lower than the models proposed by other authors. This fact may facilitate their actual application in clinical care.30
Recently, Hurria et al. have advocated the implementation of a new model of CGA within Oncogeriatrics cooperative groups.31 The use of a homogeneous model of CGA could allow a better interpretation of the results of the studies.
Screening for Frailty in the Elderly
In general, the frail elderly are the patients who could benefit most from the application of a model of CGA. But how can a frail elderly cancer patient be distinguised a priori?
For over 50 years, oncologists have used the Karnofsky scale (Karnofsky Performance Status Scale) to measure the level of activity of patients diagnosed with cancer and the care they require.32 Repetto et al. demonstrated that CGA adds additional information to the functional status, as measured, for example by performance status, in elderly patients with cancer.33
An inability to perform one or more of the basic activities of daily living (ADL) (bathing, continence, feeding, transferring, toileting, dress) classifies a person as frail, and predicts that the elderly person will have complications if subjected to any circumstance that causes stress (for example, the administration of chemotherapy).34 Likewise, inability to carry out instrumental ADL (IADL), or ADL, are associated with increased mortality.35 Furthermore, dependence on some instrumental activities predicts the development of dementia36 and an increased risk for toxicity associated with chemotherapy.37
Many authors have attempted to operationalize the concept of ‘frailty,’ and have provided a list of objective criteria. As a result, they have been proposed a set of scales and screening tools, which aim to better define and quantify this syndrome. Along with this, since a complete CGA is timeconsuming, these screening questionnaires can identify those patients who may benefit from a full CGA. Even so, it is not known which of these tools are the best to get a proper selection of the patients, and which are preferable in the field of Oncogeriatrics.
In the field of cancer in the elderly, the frailty screening questionnaires used are the Vulnerable Elderly Survey (VES-13), the Geriatric-8 (G-8), and the Groeningen Frailty Index (GFI).
The VES-13 questionnaire assesses age, functional status, self-perceived health status, and activity level of the patient.38,39 In an elderly sample from Medicare, which was used to validate this test, a score ≥3 identified as vulnerable 32% of the elderly. This group had a risk for death or functional deficit at 2 years which was four times greater compared with those who scored <3. Higher scores predicted an increased risk for death or functional decline,40 so that a score ≥3 implies that it is necessary the application of a complete CGA.39 The average time to complete this test is less than 5 minutes.41 The VES-13 has been used in oncology as a tool to select the elderly who could tolerate chemotherapy.42 Recently, Mohile et al. conducted a pilot study with elderly patients diagnosed with prostate cancer treated with androgen ablation, and correlated the VES-13 questionnaire with the conventional CGA. Patients who scored >2 in the VES-13 had worse functional status, were less fit, and had more comorbidity and poorer cognitive function.43 A study conducted in a Spanish population (sample restricted to patients diagnosed with breast cancer), showed that there is a good correlation between the VES-13 questionnaire and the CGA, and that the capacity of the questionnaire to predict frailty is high.44 Luciani et al. indicated that the VES-13 questionnaire is highly predictive of functional impairment and represents a frailty-screening tool useful in elderly cancer.45 According to Owusu et al., both the Eastern Cooperative Oncology Group PS (ECOG-PS) and the Karnosfky score are equivalent to the VES-13 questionnaire in their ability to identify patients at risk for frailty.46
More recently, the VES-13 questionnaire was compared with the Linda Fried criteria (the Cardiovascular Health Study [CHS] project), with the former demonstrating greater sensitivity than the second screening tool.47
However, although the VES-13 questionnaire is the most widespread and the most widely used as a screening tool of frailty, some authors believe that the VES-13 cannot replace or substitute the CGA, because, according to the various studies in which it has been used, its negative predictive value does not exceed 70%.48
The G8 questionnaire contains seven items that correspond to the Mini- Nutritional Assessment (MNA) and one age-related item. The cutoff point of 14 points is accompanied by 85% sensitivity and 65% specificity to predict frailty. This questionnaire, according to the authors, is a good screening tool of frailty.49 However, there are only a few studies that have used such a screening questionnaire. The GFI is a simple screening tool for frailty and, according to some authors, it is useful to select those elderly in which it is necessary to apply a CGA and to predict the risk for mortality associated with chemotherapy.50 It can therefore be seen more studies are necessary to analyze the best tool to predict risk for frailty in elderly patients diagnosed with cancer.
In a recently published study, the Study of Osteoporotic Fractures (SOF), as a predictor of frailty, was examined. Its sensitivity compared with the CGA was 89% (95% CI 84.7–92.5%) and its specificity was 81.1% (95 % CI 73.2–87.5%). The authors believe that this is another screening tool to be used in this population.51
Furthermore, the Barber questionnaire is the most commonly used in Spain in an outpatient setting.52 It consists of nine items, is easy to use, and allows elderly patients with risk for frailty to be selected (by answering ‘yes’ to one or more of its nine items). It has a high sensitivity (95%).53 Each item can be considered a risk factor for functional impairment and/ or institutionalization.
The Barber questionnaire has been used only once in the field of Oncogeriatrics. Its correlation with the CGA and its ability to predict frailty is not high.44
Finally, Hamaker et al. have conducted a systematic review of various screening questionnaires in elderly cancer patients (VES-13, Triage Risk Screening Tool [TRST], G8, GFI, Barber, Linda Fried criteria and CGA short form), and concluded that, for the moment, all elderly cancer patients should be given a complete CGA, because none of the screening tools have an adequate discriminative power.54
The purpose of these questionnaires is to evaluate elderly patients diagnosed with cancer and to select those patients who cannot tolerate chemotherapy. Today, two models for predicting toxicity in these patients are available: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) index by Extermann et al., and an index by Hurria et al. According to the CRASH index, the predictors of hematologic toxicity are the number of lymphocytes, the levels of aspartate aminotransferase (AST), IADL score, and the level of lactate dehydrogenase and diastolic blood pressure; the predictors of nonhematologic toxicity are hemoglobin level, creatinine clearance, the serum albumin level, the self-perceived health status, the baseline status (measured by ECOG-PS), cognitive status (assessed by the Mini-Mental questionnaire), and the nutritional situation (measured by the MNA).55 On the other hand, Hurria et al.’s model, which also includes geriatric assessment variables, laboratory variables and other variables related to the patient, and tumor and treatment characteristics, allow the classification of patients into low, intermediate, or high risk for toxicity of chemotherapy.56
The creation of such indices, as well as the application of a model of CGA can provide adequate care to the elderly cancer patients.
Conclusions
Research in Oncogeriatrics should focus on improving a standardized geriatric assessment in elderly patients, to increase biologic knowledge of this group of the population, and to develop specific clinical trials for the most vulnerable patients and/or those older than 75 years of age. So far, there is no an unanimous model of CGA in Oncogeriatrics, but there is sufficient evidence indicating that such an assessment should be carried out. However, despite attempts to replace the CGA by other tools which are easier and less time-consuming to be applied, at the moment, none of the questionnaires analyzed was shown to be able to replace the CGA.


