Liver cancer has contributed to an annual 30,200 deaths in the US so far in 2018.1 An overwhelming majority is due to hepatocellular carcinoma (HCC) in the setting of advanced liver disease.2 While chronic hepatitis C and alcoholic liver disease traditionally have been the most common etiologies of cirrhosis in the US, the prevalence of non-alcoholic fatty liver disease is on the rise. Chronic hepatitis B is also important among patients who have emigrated from high-risk areas, such as east and southeast Asia, Africa, and South America.
Diagnosis
The diagnosis of HCC is often made on the basis of a typical clinical scenario, such as cirrhosis from any cause, elevated α-fetoprotein, or specific radiographic criteria on multi-phase computed tomography (CT) or magnetic resonance image (MRI). The Liver Imaging Reporting and Data System (LI-RADS) provides standardized reporting criteria and categorizes a liver lesion by probability of HCC based on specific features: lesion size, growth over time, arterial phase hyper-enhancement, venous phase washout, enhancing pseudo-capsule, and invasion of portal vein.3 A LI-RADS score of LR-5 is diagnostic of HCC in the setting of either imaging-proven or biopsy-proven cirrhosis without need for further invasive biopsy of the actual liver lesion. Furthermore, des-γ-carboxy prothrombin (DCP) and lectin-reactive α-fetoprotein (AFP-L3), both of which were previously studied as serum biomarkers for HCC surveillance, have recently been shown to be associated with poor prognosis in HCC.4–6 Use of both biomarkers in a statistical model called GALAD score (age, sex, AFP, AFP-L3, and DCP) could help establish the diagnosis of HCC and even predict survival in some patients.7 The GALAD score calculator is at www.mayoclinic.org/medical-professionals/model-end-stage-liver-disease/galad. Nevertheless, biopsy of the liver lesion may be prudent in the setting of no prior liver disease or cirrhosis, only LI-RADS LR-3 or LR-4, clinical or radiographic features suggestive of intrahepatic cholangiocarcinoma, or a prior different cancer diagnosis. Furthermore, slow-growing dysplastic nodules on imaging can sometimes be mistaken for HCC, and different imaging modalities could also yield discordant LI-RADS results.8 Finally, as precision oncology research becomes more likely to link a specific class of systemic therapy to a predictive biomarker, patients will likely benefit from tumor biopsy for detailed molecular testing.
Overall management
Management of HCC relies on a unique staging system that accounts for both the status of the cancer and the extent of liver disease, with the Barcelona Clinic Liver Cancer (BCLC) staging system being used most commonly in randomized controlled treatment trials.9 Child-Pugh score, which includes serum bilirubin, serum albumin, international normalized ratio (INR), clinical evidence of ascites, and hepatic encephalopathy, is frequently calculated to assess the extent of liver dysfunction. The Model for End-stage Liver Disease (MELD) score, which uses serum bilirubin, INR, and serum creatinine, is also used to assess severity of and prognosis for liver disease.4–6,10,11 Other staging systems, such as the Tumor Node Metastasis (TNM) classification, Okuda staging, Cancer of the Liver Italian Program (CLIP) score, Chinese University Prognostic Index (CUPI), Japan Integrated Staging (JIS), Groupe d’Etude et de Traitement du Carcinoma Hépatocellulaire (GRETCH), China Integrated Score (CIS), and Tokyo score have all been validated and compared to each other with various advantages and disadvantages depending on the study population, statistical model, and relevant treatment interventionat hand.12–14 However, BCLC staging remains the most relevant for medical oncologists today.
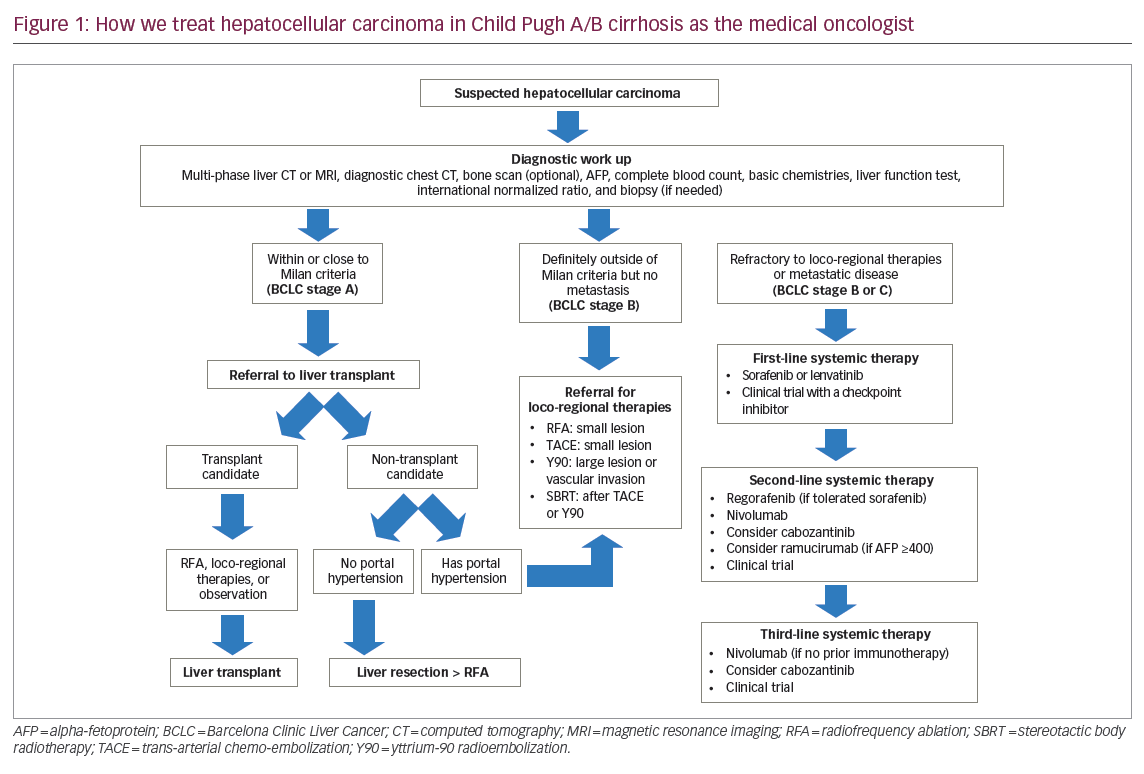
Curative treatment options include surgical resection, liver transplantation, and radiofrequency ablation, either performed intraoperatively or percutaneously. Only liver transplantation addresses both the cancer and the underlying liver disease, and typically those who meet the Milan criteria (solitary lesion of ≤5 cm or up to three lesions with the largest ≤3 cm) have the optimal recurrence-free and overall survival after liver transplantation.15 Nevertheless, a number of down-staging strategies have been developed and subsequently validated with similar benefit,16,17 and consultation by a transplantation team is still warranted in a patient whose disease seems to be outside the Milan criteria but still non-metastatic. A recent meta-analysis favors liver transplantation over liver resection due to a statistically significant disease-free survival benefit, though without a definite overall survival advantage.18 Bridging strategies using a combination of loco-regional therapies may prevent lesions from growing beyond the Milan criteria during the potentially long waiting period for an available liver transplant donor organ.19 Figure 1 describes all relevant treatments today and proposes a potential management algorithm and other factors, recognizing there could be multiple versions of standard of care according to institutional practice.
In contrast, liver resection is preferable in patients with absent or only early cirrhosis without portal hypertension and with a cause of liver disease that can be treated or even cured (such as hepatitis B or C). It is also a reasonable option in patients who decline or are deemed not a candidate for liver transplant. However, patients with significant cirrhosis have higher risk of recurrent HCC compared to those with less fibrosis,20 and liver resection does not address the underlying liver disease that continues to be a risk factor for recurrent HCC. Patients with active viral hepatitis and more dysplastic cirrhosis may thus have high recurrence rates after definitive non-transplant therapies. Radiofrequency ablation can be used alone and as an adjunctive treatment to loco-regional therapies to optimize cure rates, as well as for liver lesions not anatomically amenable to resection. Ongoing discussions with a liver transplant center are vital in discerning the specific treatment pathways (such as curative versus non-curative, transplant versus non-transplant, bridging versus down-staging) that could change for a given patient over time.
Loco-regional therapies are considered palliative due to the possibility of residual disease when managed non-operatively, and discussions with interventional radiology and radiation oncology through multi-disciplinary boards can help select patients who may derive the most clinical benefit. Some patients may gain long progression-free intervals and may be candidates for multiple subsequent liver-directed therapies. Three common options are trans-arterial chemoembolization (TACE), radio-embolization with yttrium-90 tagged glass microspheres (Y90 or TARE), and stereotactic body radiotherapy, all of which can be used individually or in combination. Y90 and stereotactic body radiotherapy are generally used for larger liver lesions while TACE is typically used for smaller lesions. Radiofrequency ablation has been used in conjunction with TACE to maximize the clinical benefit.21 A historical meta-analysis has previously noted a survival benefit with TACE,22 which has led to the development of many new loco-regional therapies. However, two recent rigorous phase III randomized controlled trials, the SIRveNIB and SARAH studies, unfortunately demonstrated no overall survival benefit for Y90 therapy when compared to sorafenib in patients with BCLC stage B who failed prior TACE.23,24 Both studies focused on patients with vascular invasion or with progression after prior TACE, and so the study populations were at higher risk for disease progression and metastatic disease compared to prior studies with TACE. Thus, Y90 therapy remains a viable option for previously untreated patient with higher tumor burden or vascular invasion.25,26 Lastly, stereotactic body radiotherapy is gaining popularity and has demonstrated survival benefit when given in sequence with TACE compared to sorafenib.27 All of these approaches are applicable to patients with non-metastatic disease and with no evidence of hepatic decompensation such as elevated liver function tests, ascites, or encephalopathy because there is a recognized risk of serious hepatotoxicity and even procedure-related mortality.
Systemic treatment
For patients with metastatic disease, vascular invasion, or progression after loco-regional approaches, several systemic therapies have demonstrated an overall survival benefit.28–32 It is important, however, to note that almost all clinical trials in this space enrolled only patients with preserved liver function and no evidence of clinical ascites or encephalopathy.28–32 In fact, end-stage liver disease rather than HCC may be the dominant contributor to morbidity and mortality in patients with BCLC stage C. The definite endpoint used in oncology drug trials for HCC has thus far been overall survival, but objective response rate by CT scan and progression-free survival are increasingly used for drug approval as well. Objective response rate as determined by modified Response Evaluation Criteria in Solid Tumors (mRECIST) has been adopted in contemporary clinical trials as a better surrogate for overall survival.33,34 mRECIST recognizes events not captured by standard RECIST, specifically tumor necrosis as a response event and infiltrative lesions, new lesions, and malignant effusions as progression events. Strict mRECIST criteria are not commonly used in clinical practice, but the key concepts can help medical oncologists decide whether to switch treatment or not.
Historically, traditional chemotherapy with doxorubicin or fluoropyrimidine has seen challenges due to risk of hepatic decompensation and myelosuppression. Of note, combination chemotherapy with cisplatin, doxorubicin, interferon, and fluorouracil had no better survival advantage than single-agent doxorubicin but had more serious toxicities.35 Subsequent systemic treatment studies have concentrated on small molecule inhibitors targeting angiogenesis and immunotherapy approaches with checkpoint inhibitors.
Table 1 summarizes the first- and second-line treatment options based on registration trials submitted to the US Food and Drug Administration (FDA) for approval. Sorafenib was the only FDA-approved first-line treatment until August 2018. Lenvatinib has also received FDA approval recently through the REFLECT trial, which has demonstrated non-inferior overall survival to sorafenib, though perhaps at the cost of higher proportion of adverse events.28 While the original SHARP trial, which led to the approval of sorafenib in 2007, and the recent REFLECT trial included almost exclusively Child Pugh A cirrhosis, clinicians today often prescribe sorafenib for patients with Child Pugh B cirrhosis and adjust the starting dose to minimize toxicity.36,37 Safety and efficacy of lenvatinib in Child Pugh B cirrhosis with HCC is not well documented. Survival benefit from first-line sorafenib was also confirmed in a phase III trial in the Asia-Pacific region, where chronic hepatitis B is the predominant cause of liver disease and HCC.38 Likewise, the REFLECT trial was an international trial that included sites in Asia-Pacific, Europe, and North America with a diverse etiology of cirrhosis.28 Thus, sorafenib and lenvatinib both represent equivalent first-line systemic treatment options for HCC. Nevertheless, it is important to recognize that a number of targeted agents in angiogenesis and cell growth pathways have not demonstrated superior survival benefit compared to sorafenib, including brivanib, everolimus, tivantinib, and sunitinib,39 further highlighting that the clinical benefit of targeted agents may only be in small subsets of patients with HCC.
Regorafenib and nivolumab are both FDA-approved in the second-line setting, but cabozantinib and ramucirumab have also recently demonstrated survival benefit in select patients (Table 1).31,32 The RESORCE study, which tested regorafenib versus best supportive care, enrolled only patients who had tolerated a sufficient sorafenib dose (≥400 mg/day for ≥20 of last 28 days) and demonstrated progression on this first-line treatment, while the single-arm CheckMate 040 study with nivolumab included patients who were intolerant or even refused sorafenib.30,40 Nivolumab demonstrated a response rate of 20% and impressive median overall survival of 15.6 months, though a minority of patients from the non-randomized phase II study had not received first-line treatment previously.40,41 The CELESTIAL study, which tested cabozantinib versus best supportive care, included patients with prior sorafenib use and patients with multiple lines of prior systemic treatment, and thus the results could be applicable in the second-line or third-line setting. Ramucirumab previously did not show a survival advantage in an unselected population in the second-line setting as per the REACH-1 study.42 However, in the REACH-2 study, patients with low AFP and Child Pugh B cirrhosis were both excluded, resulting in a modest survival benefit observed in the narrowly selected patient population.32 All of these studies, with the exception of nivolumab, demonstrated only modest overall survival benefit; therefore, it remains critical to be able to identify patients who benefit most from these new agents.
Ongoing phase III clinical trials with checkpoint inhibitors may report substantial clinical benefit and change the treatment paradigm in advanced HCC in the near future. Pembrolizumab is being studied in the second-line setting (KEYNOTE-240; ClinicalTrials.gov Identifier: NCT02702401), and a promising phase II trial (KEYNOTE-224) has already noted a response rate of 17%.43 Nivolumab (CheckMate 459; ClinicalTrials.gov Identifier: NCT02576509), durvalumab with tremelimumab (HIMALAYA; ClinicalTrials.gov Identifier: NCT03298451), and atezolizumab with bevacizumab (Imbrave150; ClinicalTrials.gov Identifier: NCT03434379) are all being tested head-to-head with sorafenib in the first-line setting with overall survival as the primary endpoint. Atezolizumab and bevacizumab have previously demonstrated synergistic clinical benefit, combining checkpoint inhibition and anti-angiogenesis in metastatic lung cancer,44 and perhaps there could be a similar interaction in HCC as well. Lenvatinib is also being studied with pembrolizumab in several early-phase trials including HCC (ClinicalTrials.gov Identifier: NCT03006926). Correlative studies with PD-L1 expression, microsatellite instability, tumor mutation load, and other potential predictive biomarkers may also play a role in treatment selection in the future. If these studies translate, the promising response rates observed in early-phase studies to a definite survival benefit compared to sorafenib, immunotherapy alone, or in combinations will likely replace small molecule inhibitors as the preferred first-line treatment option and possibly even as adjuvant treatment after surgery or ablation (CheckMate 9DX; ClinicalTrials.gov Identifier: NCT03383458). The ongoing phase III trials are noted in Table 2.
Future directions
Beyond angiogenesis and checkpoint inhibition, a number of pathways have been hypothesized to contribute to carcinogenesis of HCC and could serve as novel therapeutic targets. Glypican-3, a membrane protein constituted with heparin sulfate proteoglycan and involved in cell proliferation, is a known molecular target in HCC, and manipulation of T cell receptor to recognize glypican-3 has been the subject of ongoing investigation.45–47 Chimeric antigen receptor (CAR) T cells are currently FDA-approved for refractory aggressive B cell lymphomas and refractory B lymphoblastic leukemia by targeting CD19, a B cell specific marker. A similar treatment concept is being explored in solid tumors, and glypican-3 may be an ideal target specific for HCC (ClinicalTrials.gov Identifier: NCT02905188).
Another immunotherapy concept is the use of oncolytic viruses, namely JX-594 (pexastimogene devacirepvec, or Pexa-vec), a modified vaccinia virus, and talimogene laherparepvec (T-VEC), a modified herpes virus. Both are designed to express granulocyte-macrophage colony-stimulating factor and can cause autolysis of the tumor at sufficient infectious dose. Pexa-vec has demonstrated acceptable tolerability, clinical evidence of tumor necrosis radiographically, and biomarker evidence of prolonged anti-cancer immunity post-treatment in HCC;48 as a result, an ongoingphase III clinical trial is currently comparing sorafenib plus Pexa-vec to sorafenib alone (ClinicalTrials.gov Identifier: NCT02562755). T-VEC has demonstrated safety and durable responses in unresectable cutaneous melanoma.49 Following its FDA approval for melanoma, T-VEC is currently being clinically studied in both primary HCC and any solid tumor liver metastases, alone as single agent and in combination with checkpoint inhibitors.
The observation of HCC being more common in men has to led to interesting studies describing increased androgen receptor expression in HCC and its interactions with other established molecular pathways in HCC.50–52 These results have led to a phase II study with enzalutamide (ClinicalTrials.gov Identifier: NCT02528643) that will likely report out in the near future.
Yet another observation is that HCC cells lack specific enzymes that convert citrulline into the amino acid arginine,53 and treatment using a Mycoplasma-derived enzyme called ADI-PEG 20 to deplete arginine production has been thought to be beneficial in the treatment of advanced HCC. Despite disappointing results from a recent phase III trial that compared ADI-PEG 20 to best supportive care in advanced HCC,54 its use in combination with chemotherapy is being studied further in HCC and other gastrointestinal cancers.55
With respect to more traditional pathways of cell proliferation and invasion, the role of PI3K/AKT/mTOR pathway is well known.56 Unfortunately, everolimus has previously demonstrated no overall survival benefit in the EVOLVE-1 study.57 Even so, drug development in this space continues to improve, and a stronger mTOR inhibitor, MLN0128/INK128 (sapanisertib), has already demonstrated in vitro activity in sorafenib-resistant HCC lines.58 Inhibition of transforming growth factor beta (TGF-β) signaling, another pathway of cell proliferation, with LY2157299 (galunisertib) has also shown promising activity in vitro and is thus a candidate for clinical trials.59 TGF-β signaling has been linked to expansion of cancer stem cells, which has been proposed to be the true progenitor of HCC, and thus various mechanisms of suppressing cancer stem cells is being actively investigated in HCC.60 Furthermore, inhibition of c-MET alteration, which has a direct correlation with aggressiveness of HCC and progression of cirrhosis, has been tested with multiple small molecule inhibitors.61 Cabozantinib, as a non-selective tyrosine kinase inhibitor with multiple targets including c-MET, has demonstrated clinical benefit in the second-line setting. However, a more selective c-MET inhibitor, tivantinib, did not demonstrate overall survival benefit in the METIV-HCC trial.62 Activated fibroblast growth factor receptor pathway is yet another pathway important in cell proliferation and angiogenesis of HCC in both cell lines and animal models,63,64 and a number of small molecular inhibitors including BLU9931 and H3B-6527 have demonstrated promising pre-clinical activity.65,66 Development of more potent small molecular inhibitors for each of these well-known pathways remains important despite previous setbacks seen with the first generation of targeted drugs.
Exciting research in HCC will continue to bring new drugs targeting various hypothesized pathogenic mechanisms in HCC, some more traditional and some quite innovative. Hepatotoxic side effects and management of irreversible cirrhosis will remain important clinical issues to address for any novel systemic therapy. Prevention and treatment of hepatitis B and C may shift the heterogeneous biology of the current study population, and how future patients with HCC will respond to each of these new therapeutic strategies will continue to evolve as well.


