In December 2019, an outbreak of pneumonia of unknown aetiology was observed in the Chinese city of Wuhan, capital of Hubei province. By January 2020, this outbreak was attributed to a novel virus classified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the latest member of the coronaviridae family responsible for major infectious outbreaks, including severe acute respiratory distress syndrome (SARS) in 2002–2003, and Middle East respiratory syndrome (MERS).1 Unlike the preceding outbreaks, coronavirus (COVID-19) rapidly spread throughout the world, reaching 11 million people by July 2020, with 570,000 associated deaths, with current incidence data (as of September 2021) of over 229 million cases and 4.7 million deaths worldwide.2
Clinically, COVID-19 displays a wide spectrum of illness, with most cases resulting in asymptomatic disease, while others require hospitalization or management in the intensive care unit (ICU) for acute respiratory distress and end-organ failure. Since the onset of the global pandemic, various reports of abnormal haematological parameters and clinical sequelae of thrombosis have been reported. Early reports of critically ill patients with severe COVID-19 showed a rate of pulmonary embolism (PE) as high as 40% of patients.3 Strikingly, rates of increased thromboembolism were also reported despite the widespread use of prophylactic and therapeutic anticoagulation in hospitalized patients.4 The unique pathobiology, as well as optimal prevention and treatment of thrombosis in COVID-19, remain largely uncertain and controversial at this time.
The aim of the current study was to highlight key pathophysiological mechanisms of COVID-19-associated coagulopathy and to summarize incidence rates of venous and arterial thrombotic events, comorbidities conferring risk and current treatment guidelines, including data from ongoing clinical trials.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) recommendations were followed for this review. An electronic search was performed of medical literature published between 1 December 2019 and 30 September 2021 within PubMed and Web of Science databases using the following Medical Subject Headings terms (including variations, synonyms and acronyms): ‘COVID-19’, ‘COVID’, ‘SARS-CoV-2’, ‘coronavirus’, ‘coagulopathy’, ‘endothelial dysfunction’, ‘thrombosis’, ‘stroke’, ‘immunothrombosis’. References and citation lists of selected articles and reviews were also reviewed for any other relevant literature. Additionally, guidelines from relevant organizations and recent clinical trials were accessed as cited electronically to provide an up-to-date summary of recommendations.
Inclusion criteria were: 1) clinical and translational studies pertaining to coagulopathy in COVID-19 patients including case–control studies, cohort studies, observational studies and randomized clinical trials; 2) studies describing coagulation laboratory parameters or clinical outcomes.
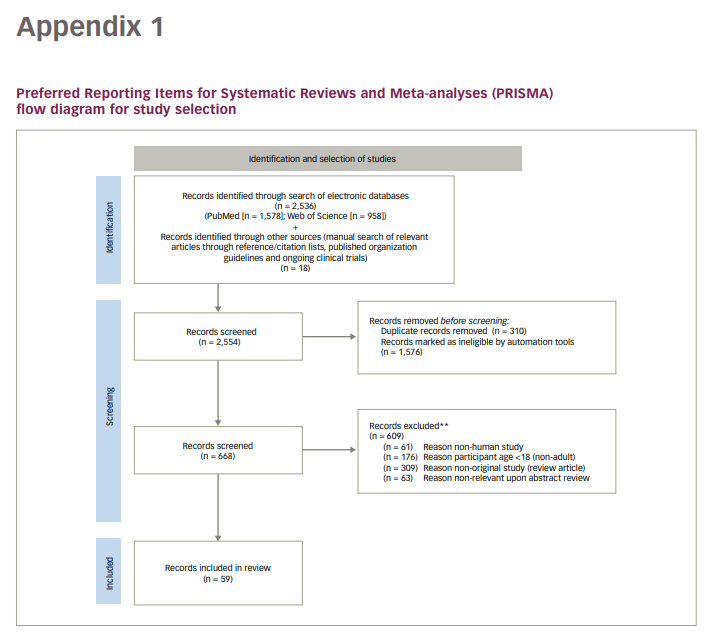
Exclusion criteria were: 1) non-English literature; 2) non-human studies; 3) patient age <18 years; 4) non-original studies (i.e. reviews, commentaries). See Appendix 1 for the PRISMA flowchart illustrating the identification and selection of studies.
Haematological abnormalities
COVID coagulopathy describes the increased rate of localized and systemic coagulation defects and associated thrombotic events observed in infected individuals. Due to the unique pathobiology and clinical sequelae of COVID coagulopathy, some authors have termed this a new disorder: COVID-19-associated haemostatic abnormalities (CAHA).5 While coagulopathic features typical of disseminated intravascular coagulation are evident, hyperfibrinogenaemia typically seen in CAHA is a prominent feature of the disease.
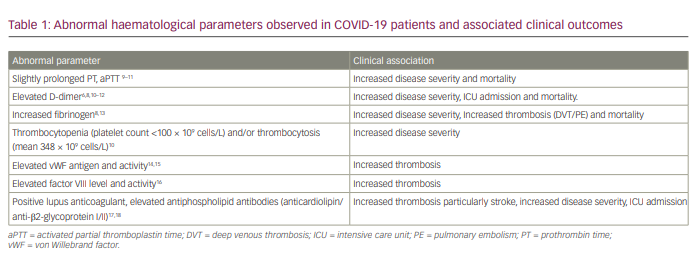
Initial reports of abnormal coagulation parameters were first noted by treating physicians in Wuhan, China. One early retrospective epidemiological report analysed 99 hospitalized patients in January 2020, and identified defective coagulation parameters that manifested as mildly elevated prothrombin time (PT) in ~5% and activated partial thromboplastin time (aPTT) in ~6%, occurring in conjunction with elevated D-dimers in the larger subset (36%).3 This was followed by another retrospective cohort study that reviewed 191 patients from two main hospitals in Wuhan, identifying a subset with disordered haematological parameters and an increased mortality rate of 28% (54 patients).6 Haematological factors associated with increased mortality included: 1) elevated D-dimer >1 ug/mL (odds ratio [OR] of 18.42, 95% confidence interval [CI] 2.64–128.55; p=0.003); 2) an increased PT (OR 4.62, 95% CI 1.29–16.50; p=0.019); and 3) increased serum IL-6 (OR 1.12, 95% CI 1.03–1.23; p=0.008).6 Another retrospective chart review of a large cohort of 1099 hospitalized patients in mainland China further highlighted the frequent presence of increased D-dimer (>0.5 ug/mL) in 46.4% of patients, associated with the primary endpoint(s) of increased need for ICU admission, mechanical ventilation, severe COVID-19 infection and death.7 Thrombosis rate was not characterized in this study.7 A large retrospective cohort study enrolling 1643 patients admitted to one of two ‘instant hospitals’ constructed in Wuhan also found that elevated fibrinogen (>420 mg/dL) was associated with a greater risk of developing critical illness (OR 2.16, 95% CI 1.05–4.46; p=0.038) and mortality (hazard ratio 4.79, 95% CI 1.14–20.20; p=0.033).8 Since these initial findings were reported, several other haematological defects have been observed in COVID-19 patients, as summarized in Table 1.8-18
Clinical thrombohaemorrhagic defects
Abnormalities in haematological laboratory parameters are associated with disease severity and mortality, and increased rates of thromboembolism reinforced the concept of a clinically distinct hypercoagulable state accompanying COVID-19 infection. Clinically relevant haemostatic defects are less frequent, and typically present with laboratory features usually seen in disseminated intravascular coagulation, such as hypofibrinogenaemia, severe consumptive coagulopathy and thrombocytopenia. Despite differences in study design, cohort selection and definable endpoints, numerous studies have identified increased rates of both venous thromboembolism (VTE) and arterial thromboembolism. VTE complications include deep venous thrombosis (DVT) and PE, while arterial thromboses include stroke, myocardial infarction (MI) or atypical thrombotic sites (e.g. iliac artery). A summary of the findings from reviewed reports is shown in Table 2.10, 17–25
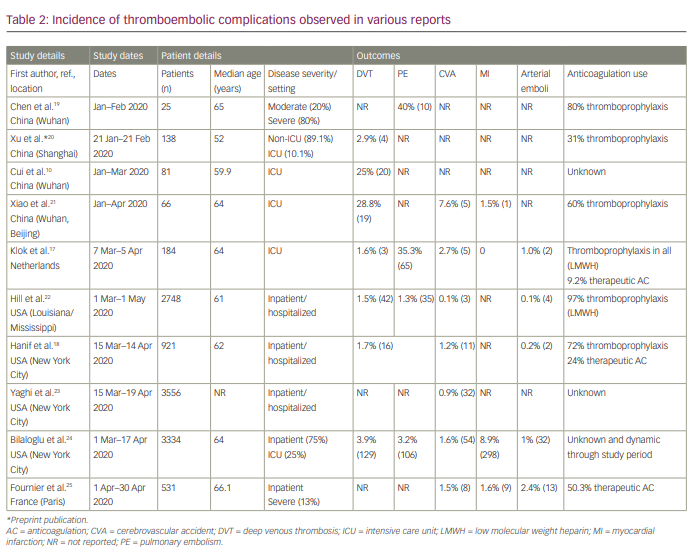
Rates reported in early experiences in Wuhan for critically ill patients in ICU approached 40% for PE and 28.8% for DVT.19,21 Subsequent studies focusing on hospitalized patients in the USA have shown much lower rates of PE (1.3%) and DVT (1.5%).22 A large meta-analysis by Jimenez et al. pooling data of 20 studies (aggregate 9350 non-ICU hospitalized patients) identified an overall VTE incidence of 7.1%.26 Stroke was reported in 7.6% of patients in an early study from mainland China and Wuhan, although comparatively lower levels have been identified in the USA and Europe, with rates of 0.1–2.7%.17,18, 21–25
Pathophysiology
Various mechanisms have been proposed as contributing to the development of the haemostatic abnormalities seen during COVID-19 infection. The findings are summarized in Figure 1.
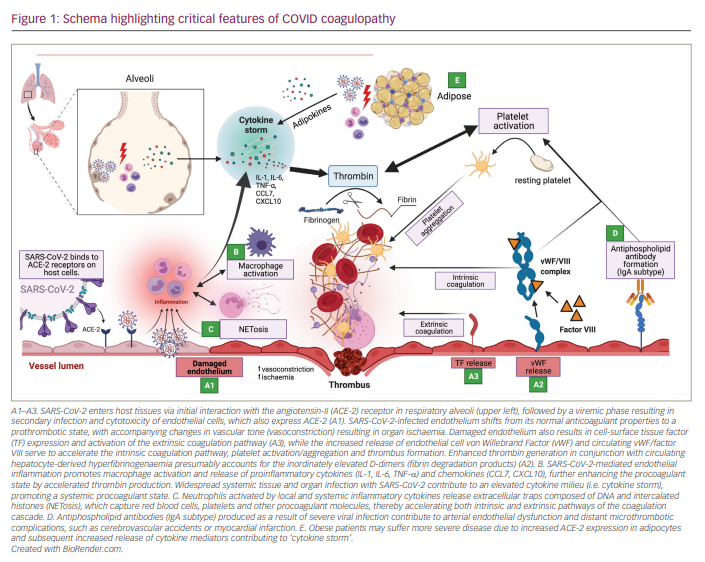
Endotheliopathy (A in Figure 1)
Vascular endotheliopathy has been proposed to be of central importance in CAHA. The vascular endothelium plays an important role as a paracrine, autocrine and endocrine organ regulating vascular tone and homeostasis. Shifts in vascular endothelial tone due to infection result in increased vasoconstrictor activity, which may result in organ ischaemia, inflammation and ultimately a procoagulant state. COVID-19 has been shown to infect engineered human blood vessels in vitro.27 Clinicopathological autopsy series have demonstrated the presence of endotheliitis and intracellular viral inclusions across vascular beds of different organs (heart, small bowel, lung).28 Of note, direct endothelial infection with SARS-CoV-2 is currently debated, with some studies showing low basal angiotensin-II (ACE-2) receptor expression in vitro via transcriptome and epigenetic data even with exposure to cytokines associated with SARS-CoV-2 infection.29 Other studies, however, have shown localization of SARS-CoV-2 in the endothelium of infected mice and non-human primates at the RNA and protein level, as well as through immunogold labelling and electron microscopic examination.30 Failure to identify ACE-2 localization in the endothelium in vitro may be explained by differences between the endothelial monolayer culture commonly used in the laboratory and the in vivo endothelial layer lining the blood vessels due to sheer stress activation by cytokine storm or tight contact with the epithelium in certain organ capillary beds such as the lungs.31
A cross-sectional study of 68 adult COVID patients (48 ICU, 20 non-ICU) compared with 13 non-hospitalized asymptomatic controls established that both ICU and non-ICU patients had elevations in plasminogen activator inhibitor-1 (PAI-1), a potent endothelial regulator of fibrinolysis. Additionally, elevations were noted in von Willebrand factor (vWF) antigen and functional activity, and factor VIII activity, with subsets of ICU patients having vWF activity above the upper limit of detection. Similarly, D-dimer and thrombin–antithrombin complexes were elevated in the aggregate cohort, with significantly higher values in the ICU subgroup. P-selectin (a marker of endothelial and platelet cell activation) and soluble CD40 ligand were also increased in ICU patients compared with controls.32 These findings provide biochemical evidence towards the presence of endotheliopathy resulting in augmented vWF release and platelet activation as contributory mechanisms in the development of COVID coagulopathy.
Inflammation and the role of macrophage activation (B in Figure 1)
Hypercytokinemia and hyperferritinemia (500–3000 ng/mL) are prominent laboratory features of severe pulmonary disease (acute respiratory distress syndrome) seen in severely ill COVID-19 patients. These findings implicate both cytokine release syndrome and features of macrophage activation syndrome (or secondary haemophagocytic lymphohistiocytosis [HLH]) as prominent pathophysiological entities. Multiplex screening for 48 cytokines performed on 53 clinically moderate/severe patients have identified monocyte/macrophage-associated cytokines CXCL10 (IFNg-inducible protein-10), CCL7 (monocyte chemoattractant protein-3), and IL-1 receptor antagonist to be at continuously high levels and associated with increased lung injury, viral load and fatal outcomes.33 Predicated on these findings, the previously validated risk calculator (HScore) has been proposed as a means of deciding which patients should be diagnosed with and treated for HLH,34 although its inadequate sensitivity and specificity in COVID-19 may be insufficient for defining the subsets requiring ICU admission or those at increased risk for death.35 Ultimately many patients fail to fulfil diagnostic criteria for HLH, most commonly due to inconsistencies related to cytopenias, hepatosplenomegaly or hypofibrinogenaemia. Furthermore, while not an obligate criterion for diagnosis of HLH, there appears to be a lack of haemophagocytosis involving bone marrow biopsies of investigated patients.36 Nonetheless, recognition of a hyperinflammatory state in a subset of COVID-19 patients with severe illness has resulted in the investigation and use of certain immunosuppressant agents, including corticosteroids, intravenous immunoglobulin, IL-1 inhibition (anakinra), IL-6 inhibition (tocilizumab) and, more recently, Janus kinase inhibition (baricitinib, tofacitinib).37-41
Neutrophil extracellular traps (C in Figure 1)
Recent studies have elucidated the role of activated neutrophils in the formation of arterial and venous thrombi. One key hallmark of the prothrombotic neutrophil is the release of web-like structures composed of DNA intercalated with histones and granule proteins (referred to as neutrophil extracellular traps [NETs]). Intravascular NETs have been characterized and isolated from the pulmonary vasculature, kidney and heart of COVID-19 patients. Elevated intravascular NETs were also found to correlate with severe disease. By providing a scaffold, NETs capture platelets, red blood cells and other procoagulant molecules and thereby activate both the intrinsic and extrinsic coagulation cascade.42 In a study by Nicolai et al., platelets from patients with severe COVID-19 showed increased adhesion to neutrophils and enhanced NET formation, supporting a mechanistic link for immunogenic platelets driving neutrophil activation.15
Antiphospholipid antibodies (D in Figure 1)
Antiphospholipid antibody syndrome is an autoimmune disorder characterized by the presence and persistence of antiphospholipid antibodies (aPLs), and accompanied by clinical sequelae of arterial and venous thrombosis and/or recurrent foetal loss. An increasing incidence of aPLs in association with thrombotic sequelae has been found in COVID-19, particularly as a risk factor for cerebral infarction. In one report, three patients suffered multiple cerebral infarctions associated with the detection of IgA anticardiolipin aPLs, and IgA and IgG beta 2 glycoprotein I antibodies.43 Another recent cohort study identified aPLs in 47.0% (31 of 66) of patients using a cutoff of >20 chemoilluminescent units and in 31.8% (21 of 66) of patients when using a higher cutoff of >40 units.21 All patients found to have aPLs were noted to be critically ill (admitted to ICU). In another study, aPLs were found in the majority of patients admitted to the ICU (87.7% of 150 patients).44 Interestingly, IgA isotype (most commonly associated with mucosal infection) aPLs were the most common in COVID-19 patients and are also significantly and independently associated with acute MI and acute cerebral ischaemia.
Obesity and adipose tissue signalling (E in Figure 1)
Obesity has been increasingly recognized as a determinant of severe disease and worse outcomes in patients infected with COVID-19.45 Adipose cells have also been characterized to express ACE-2 (the primary extracellular entry mode for COVID-19 virions), and obesity has been shown to increase its expression. Furthermore, adipose tissue has previously been shown to have a role in regulation of inflammation through elaboration of cytokines IL-6, tumour necrosis factor-a (TNF-a), macrophage chemoattractant protein-1, and its reservoir of both paracrine/endocrine hormone mediators.46 Local release of such factors may explain sites of ‘abnormal’ thrombosis such as cerebrovascular accident (CVA), MI in young patients, or why this subset of patients experiences worse end-organ damage and ultimately mortality. Systemic release of such adipokines may mediate vascular thrombosis through promotion of Virchow’s triad in the form of increased venous stasis, endotheliopathy and release of hypercoagulable proteins (soluble urokinase plasminogen activator receptor and/or PAI-1).47 Ultimately further studies are necessary to completely characterize the complex interplay between obesity and COVID-19.
Management
Laboratory biomarkers in disease prediction and prognostication
As shown in Table 1, several haematological parameters have been found to be abnormal with COVID-19 infection and may correlate with disease severity or as predictors for clinical outcomes, such as need for mechanical ventilation, ICU admission or mortality. At this time, prospective trial-based evidence is lacking and the utility of such parameters in clinical decision making remains uncertain. Based on societies including the American Society of Hematology (ASH) and the National Institutes of Health (NIH), current recommendations advocate for measurement of platelet counts, PT, aPTT, fibrinogen and D-dimer in inpatients as proposed prognosticators, but do not recommend sole use of laboratory tests to guide clinical imaging or treatment for VTE.48,49 Similarly, laboratory testing is not recommended in asymptomatic outpatients and should not be used to decide level of care (admission to inpatient or transfer from inpatient to ICU).49
Current recommendations for management/treatment interventions
Several organizations have published guidelines and interim recommendations for the management of thromboprophylaxis and VTE treatment in the inpatient setting, critical care setting and upon discharge.,,,, To date, there are no clear indications for prophylactic anticoagulation in non-hospitalized cohorts. Current practice guidelines for hospitalized patients, including inpatients, critically ill ICU patients and post-hospital discharge, are summarized in Table 3.48-52
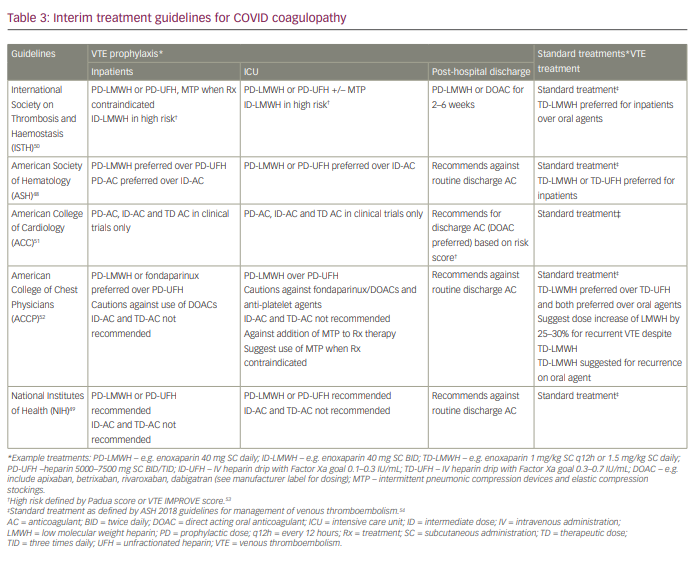
Inpatient setting
Unless there are contraindications, recommendations include the use of prophylactic dose anticoagulation for the prevention of VTE in hospitalized patients. Heparin-based anticoagulation using either low molecular weight heparin (LMWH) or unfractionated heparin (UFH) is preferred over direct acting oral anticoagulants (DOACs) or vitamin K antagonists due to risks of impaired metabolism or excretion in the setting of end-organ dysfunction (hepatic, renal), which often accompanies severe COVID-19 infection. The International Society of Thrombosis and Haemostasis (ISTH) recommends the use of higher doses of anticoagulation (termed ‘intermediate dose’) for high-risk patients as defined by VTE risk prediction models such as Padua or IMPROVE.53 This diverges from the remaining agencies, which recommend against the use of higher doses of anticoagulation outside of randomized controlled clinical trials and in the absence of high-quality evidence (see Table 4 and Ongoing clinical trials, below). Additionally, all organizations recommend mechanical thromboprophylaxis be used when pharmacological anticoagulation is contraindicated due to high risk for bleeding. Of note, guidelines have not been updated to reflect interim results of ongoing clinical trials (discussed in more detail below), which suggest possible improved outcomes with the use of therapeutic dose anticoagulation for thromboprophylaxis in moderately ill hospitalized inpatients.55
Intensive care unit setting
Uniformly similar guidelines exist regarding thromboprophylaxis strategies in the critical care setting. Prophylactic dose anticoagulation is recommended in all critically ill patients (unless contraindicated) using LMWH or UFH over DOACs. Intermediate and therapeutic dose anticoagulation are generally not recommended because of concerns over excess bleeding and are reserved for clinical trials at this time. Comparable to current recommendations in moderately ill hospitalized patients, the ISTH recommends intermediate dose anticoagulation in high-risk patients based on VTE risk predictions models (Padua/IMPROVE).49,52 Importantly, preliminary data from recent ongoing clinical trials have supported the lack of benefit and possible harm with the use of therapeutic anticoagulation in this setting.54
Outpatient post-hospital management
Post-discharge anticoagulation remains controversial at this time and is an active area of clinical studies. ISTH and the American College of Cardiology recommend possible discharge anticoagulation, whereas ASH, the American College of Chest Physicians and the NIH recommend against its use.47-51
Treatment of suspected or proven venous thromboembolism
Recommendations for treatment of documented or high-suspicion venous or arterial thrombosis remain clearer, and all organizations recommend the use of established ‘standard’ treatment (non-COVID) guidelines for all hospitalized patients.47-51, 53
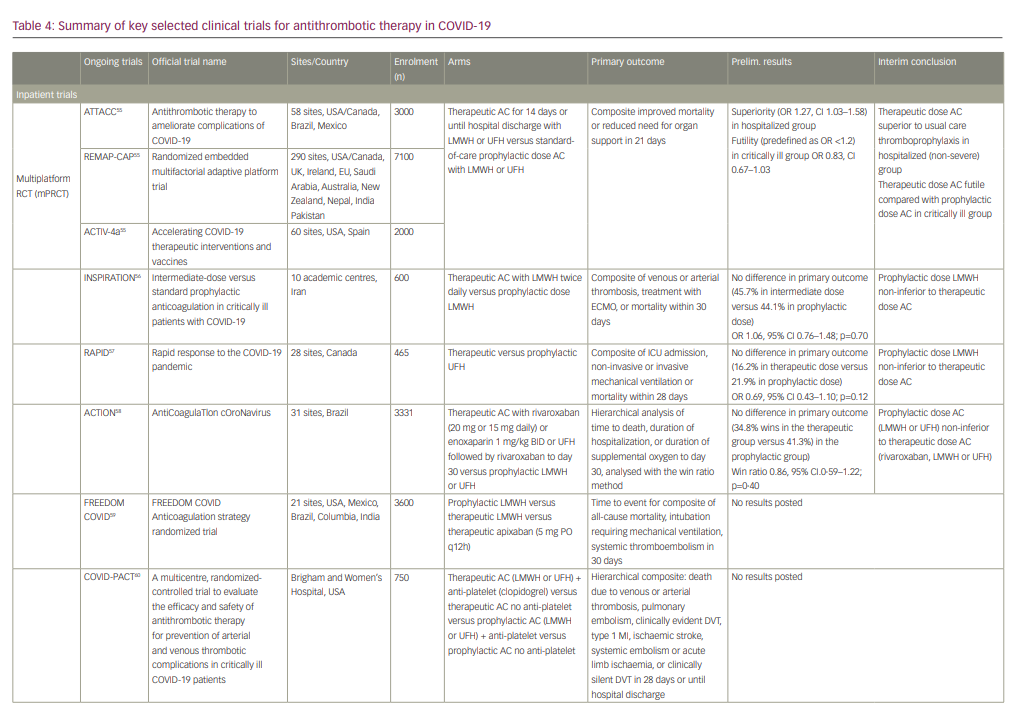
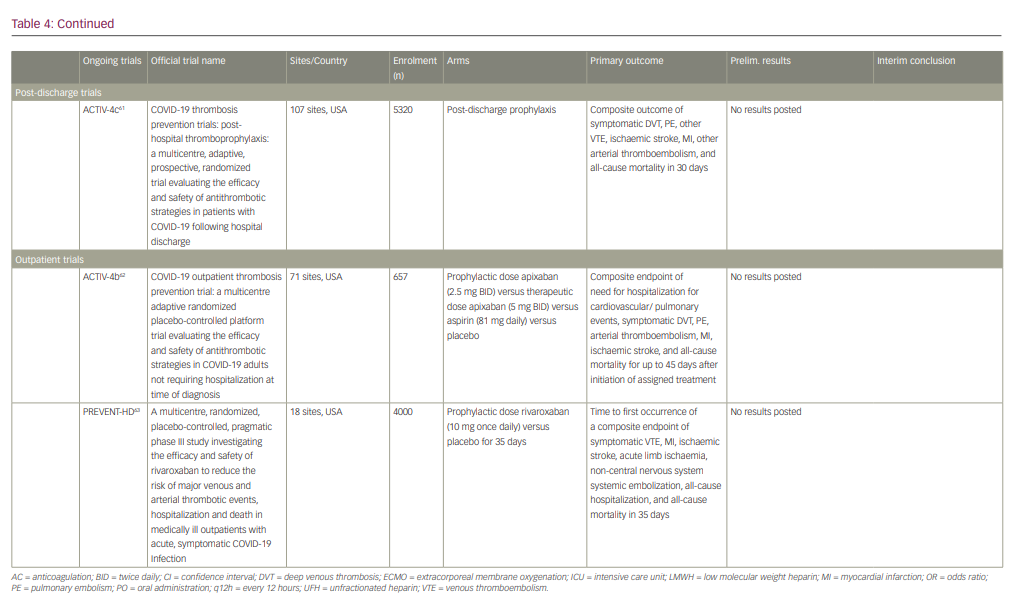
Ongoing clinical trials
Ongoing randomized clinical trials continue to investigate the safety and efficacy of various thromboprophylaxis strategies in the inpatient and outpatient setting (summarized in Table 4).55-63
At the time of writing, interim data are available from several studies including a collaborative international multiplatform randomized control trial (mpRCT) incorporating data from three studies: Antithrombotic therapy to ameliorate complications of COVID-19 (ATTACC), Accelerating COVID-19 therapeutic interventions and vaccines-4 (ACTIV-4a), and Randomized, embedded, multifactorial adaptive platform trial for community-acquired pneumonia (REMAP-CAP).55 This mpRCT seeks to compare the efficacy of therapeutic dose anticoagulation versus prophylactic dose anticoagulation in improving mortality or need for organ support in hospitalized COVID-19 patients classified as moderately or critically ill. As of December 2020, all three trials ceased enrolment of critically ill patients due to safety concerns (pre-defined futility), and also stopped enrolment of moderately ill patients after meeting the pre-specified criteria for superiority in this group.55 Interim conclusions based on pooled data analysis demonstrated superiority in the therapeutic dose anticoagulation arm compared with the prophylaxis arm in moderately ill COVID-19 patients (OR 1.27, 95% CI 1.03–1.58) but also met the pre-defined criteria for futility (OR <1.2) in the critically ill group (OR 0.83, 95% CI 0.67–1.03).55 The majority of these studies are actively recruiting, and firm conclusions await their completion.
Conclusions
In summary, SARS-CoV-2 infection often presents with a distinct form of coagulopathy responsible for significant morbidity and mortality in afflicted patients. Laboratory elevations in coagulation parameters such as D-dimer, fibrinogen, vWF antigen/activity have shown to serve a predictive role in forecasting disease severity or increased rates of thrombosis, and a prognostic role in association with increased mortality. Unique pathophysiological mechanisms have been identified as contributing to the development of COVID coagulopathy. SARS-CoV-2 entry via ACE-2 receptors precipitates a potent host immune response, endothelial damage, and activation of tissue macrophages and neutrophils leading to a prothrombotic state.
Observed rates of thromboembolic complications such as DVT, PE, MI and CVA have varied significantly during the pandemic, with notable improvement after the implementation of increased use of thromboprophylaxis during inpatient care. While several interim guidelines have been proposed by major organizations, the optimal anticoagulant dose for thromboprophylaxis in the inpatient (non-ICU) and critical care settings is currently not well established pending the results of several key randomized clinical trials, which are actively recruiting patients.