Thyroid cancer is the most common endocrine malignancy, and has increased in incidence in recent decades.1–3 Fine-needle aspiration cytology (FNAC) is the mainstay of assessment of thyroid nodules and diagnosis of malignancy.4,5 Several reports have suggested that ultrasound (US)-guided FNAC has many advantages over palpation-guided biopsy. It is now generally recommended that thyroid nodules should be biopsied under US-guidance to improve diagnostic accuracy (British Thyroid Association Guidelines for the Management of Thyroid Cancer 2014).6–8
Low adequacy rates of FNAC for thyroid nodules remains an issue, particularly in low-volume thyroid units with multiple clinicians performing this investigation. This leads to repeated FNACs and delays in diagnosis. Several strategies have been used to try to improve the diagnostic yield.9
It was apparent that insufficiency rates of thyroid FNACs were high in Northumbria Trust, leading to increased patient hospital attendance, anxiety, delay in diagnosis and treatment. These audits were undertaken to assess whether surgeon-performed US (SUS)-guided FNAC could improve the adequacy rates of thyroid nodules FNAC and shorten the time to diagnosis in a low-volume thyroid center.
Methods
In the first period, the pathology department provided us with a list of all thyroids FNACs performed in the trust by a diverse group of clinicians
and radiologists from October 2008 to April 2010, and we retrospectively reviewed the case notes and clinic letters to ascertain other clinical information. The second period included only those FNACs carried out by a single surgeon (SRA) under US-guidance from July 2013 to November 2014 and data were recorded prospectively.
In the first period, a diverse group of FNAC techniques were performed either free hand or under US-guidance, using needles of different calibres, with or without suction according to the preference of the clinician. In the second period a standard technique was employed using a Sonosite EdgeTM US system (FUJIFILM SonoSite, Inc., Washington, US) with 13–6 MHz transducer. FNAC was obtained using 21 G needle without suction, inserted into the nodule under US-guidance with rotation and multiple passes (>10). Aspirates were expelled and smeared onto glass slides and air dried or fixed in alcohol prior to cytopathology analysis according to Royal College of Pathologists guidelines.10
Cytopathology analysis was undertaken by several pathologists in Northumbria Healthcare NHS Foundation Trust (NHCT). Statistical analysis was carried with GraphPad PrismTM (GraphPad Software, Inc., California, US) and p<0.05 taken as significant.
Results
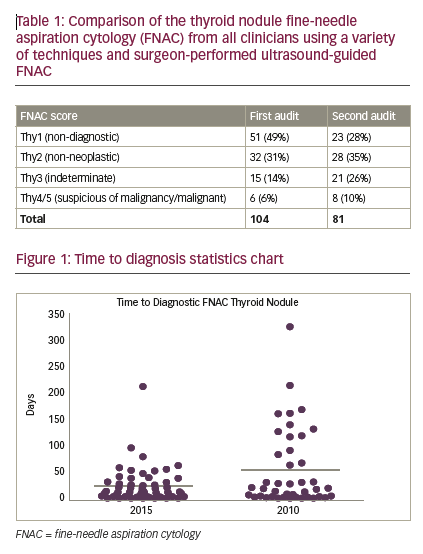
From 85 patients, 104 FNACs were identified in the first period and 81 FNACs from 49 patients were identified in the second. For nondiagnostic FNAC (Thy1) there was a statistically significant reduction of non-diagnostic FNAC rates in the second period: 23/81 (28%) versus 51/104 (49%) in the SUS-FNAC group (Fisher’s exact, p=0.0063). For non-neoplastic FNAC (Thy2), scores remained comparable: 28/81 (35%) versus 32/104 (31%). For indeterminate FNAC (Thy3), there were more Thy3 results: 21/81 (26%) versus 15/104 (14%) in the SUS-FNAC group. However, this difference just failed to reach statistical significance (Fisher’s exact, p=0.0615). For suspected malignancy/malignant FNAC (Thy4/5), while the proportion of Thy4 and Thy5 results in the SUS-FNAC group increased: 8/81 (10%) versus 6/104 (6%), overall numbers of suspicious or malignant FNAC remained low. Time to diagnostic FNAC—defined as time from decision to perform first FNAC to diagnostic (Thy2 or above) FNAC report—was significantly shorter in the SUS-FNAC group 24.2 ± 4.5 versus 54.9 ± 11.4 days (p=0.01, unpaired t-test) (see Figure 1).
Discussion
FNAC is a simple and safe procedure that has reduced the proportion of patients undergoing unnecessary thyroidectomy, while serious adverse events are rare. This was illustrated in a systematic review of 13 studies including 18,156 individuals undergoing thyroid biopsy.11 When taking a FNAC, it is important to avoid a bloody specimen as blood may obscure the thyroid epithelial cells and make cytological interpretation difficult.
Ideally, the overall accuracy of FNAC should exceed 95%, with a falsenegative rate for a benign interpretation between 0 and 3%.9,12 Repeat FNAC reduces the false-negative rate and in one series patients with benign cytology had a 90% probability of benign disease, when confirmed with repeat FNAC, this probability increased to 98%.12,13
The positive predictive value of a malignant cytology is 97–99%, as was shown in a report of 3,589 nodules from 2,587 patients evaluated and aspirated under thyroid US-guidance.14 In this study, the predictive value of a malignant versus non-malignant cytological diagnosis was 97% and 99.7%, respectively. In a similar report of 4,703 FNAC samples, the sensitivity and specificity of thyroid FNAC for the diagnosis of malignancy were 94% and 98.5%, respectively.15 Hence, the accuracy and clinical utility of thyroid FNAC is excellent. However, Janczak et al. found that, based on 108 thyroid cancers resected with 66 FNA during a 20-year period, only 14 FNA biopsies (21%) revealed cancer, all of which were confirmed in the postoperative specimen, although six cases of FNA-diagnosed cancer revealed a different histological type postoperatively.16
The non-diagnostic FNAC (Thy1) rate varies from 3% to 10% in experienced centers. The absence of malignant thyroid epithelial cells should not be interpreted as an absence of malignancy in the nodule if there are insufficient thyroid epithelial cells present in the aspirate. The Royal College of Pathologists’ guidelines recommend that “there should be at least six groups of thyroid follicular epithelial cells across the slides containing at least 10 well-visualized thyroid epithelial cells”.10 If repeated inadequate FNACs are obtained, it is recommended to repeat the FNAC under USguidance according to the American Thyroid Association (ATA) guidelines.17 A cutting needle (core) biopsy under US-guidance may also help if repeated FNAC remain insufficient for diagnosis.17
In contrast, other studies found higher insufficiency rates of approximately 15–20% for percutaneous thyroid FNACs. The highest frequency of nondiagnostic cytology occurs in cystic nodules and in very small nonpalpable nodules.18,19 The diagnostic yield of sampling palpable nodules is
enhanced when US-guidance is employed, especially when a nodule has undergone cystic deterioration. If initial US-guided FNA is non-diagnostic, a repeat US-guided FNA will yield a diagnostic cytology specimen in 75% of solid and 50% of cystic nodules.18 If there is very sparse or no aspirated material, using a larger lumen needle may result in a satisfactory specimen.17 US-guidance is preferred to palpation-guided FNAC, as the use of US improves the cytologic diagnostic accuracy rate and reduces the non-diagnostic rate.20–23
In low volume thyroid centers, due to the small number of thyroid nodule FNACs, it is impractical to have instant cytological reporting of thyroid FNACs to assess for adequacy of follicular epithelial cell numbers, due to the low numbers of cases seen, though this has shown to be a useful adjunct to FNAC to minimize inadequacy rates in high-volume centers.24 Concentrating the workload into the hands of dedicated, interested clinicians and pathologists may improve the outcome of FNAC.25,26 Multiple authors are now in favor of surgeon-performed US-guided FNACs as this has proved to provide many advantages, especially in shortening the time to diagnosis and expediting management,27,28 which is in line with our findings in this study.
Regarding the technique, samples of thyroid tissue may be obtained percutaneously with a narrower-gauge needle than that used for SUSguided FNAC, such as 25 or 27 gauge, to obtain cells for cytological analysis. Alternatively, some clinicians prefer to use a cutting needle to obtain a core of tissue for histopathological study. It should be noted, however, that there may be more complications, such as hematoma, when a core specimen is obtained.29 FNAC is therefore preferred to core needle biopsies at most centers. The fine-needle technique is popular because it is low risk and cytology provides diagnostic information about nuclear and cytoplasmic detail that is not available with a core biopsy.30
The technique for US-guided fine-needle thyroid biopsy involves three steps: puncture of the nodule or goiter, obtaining the sample, and preparation of the slides. With the surgeon performing the biopsy, results
from some studies showed that it is technically feasible,22 increases adequacy and efficacy, especially with the presence of immediate cytopathology assessment,24,26 and shortens time for diagnosis of thyroid nodules, which has implications for oncological benefit as well.28 Hilmi Bozkurt et al. reported that US-guided FNAC can be performed with a low non-diagnostic rate as experience grows.31
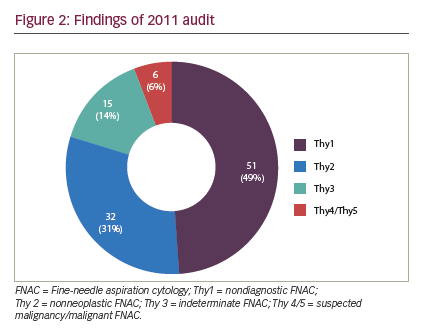
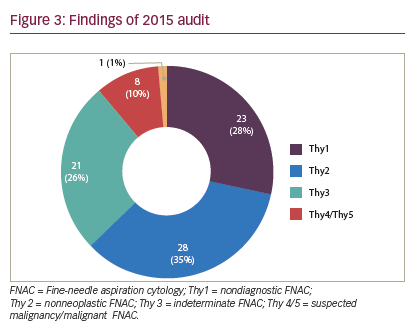
The following limitations were inherent in this study and may have biased the results. The first audit cycle demonstrated a very high rate of insufficiency in thyroid FNAC (higher than that reported in the literature) (see Figure 2). We think the reason our non-diagnosis rate was so high in the first period was that multiple clinicians were involved in performing a small number of thyroid FNAC, with or without SUS, in a low-volume thyroid center, which seems to lead to high non-diagnosis rate. Concentrating thyroid FNAC in the hands of dedicated, interested clinicians who audit their results seems to reduce our non-diagnosis rate and time to diagnosis. Although the second period did significantly improve the adequacy of FNAC, and shortened time to diagnosis, the inadequacy rate remained high (28%), suggesting that there is still considerable room for improvement (see Figure 3). However, there were clear benefits to service delivery from the use of SUS FNAC. Results from the first period were collected retrospectively and so are inherently less accurate than the prospectively collected data from the second cycle. Thyroid nodules in the second period were referred to a surgeon for assessment, so may represent a different spectrum of pathology to those assessed by all clinicians in the first period. The surgeon may have sent potentially difficult cases to radiology for FNAC in the second period, thus biasing the results. The first period also included FNACs taken free hand, which has been shown to be inferior to US-guided FNAC.
Conclusion
SUS-guided FNAC for thyroid nodules is a safe and simple technique. This study demonstrates that it leads to improved patient care by reducing inadequacy rate and shortens time to diagnosis in a low-volume thyroid center.


