Prostate cancer is the most common noncutaneous malignancy. It is the sixth leading cause of cancer-related death in men worldwide,1 and the second most common cause of cancer death in US men.2 In 2012, it was estimated that 241,740 cases would be diagnosed and 28,170 would die of the disease.3 Prostate cancer responds to androgen-deprivation therapy (ADT). However, most cases become refractory after 1 to 3 years and resume growth despite hormone therapy, leading to the state of castration-resistant prostate cancer (CRPC), for which the prognosis is poor.4 Many patients enter the disease at an early stage when the only sign of resistance to ADT is a progressive elevation of prostate-specific antigen (PSA). Progression to CRPC occurs via reactivation of androgen receptor (AR) activity,5 alternative mitogenic growth factor pathways,6 stress-induced survival genes7 and cytoprotective chaperone networks.8 Following CRPC development, the patient population is likely to continue progression to metastatic disease (mCRCP).
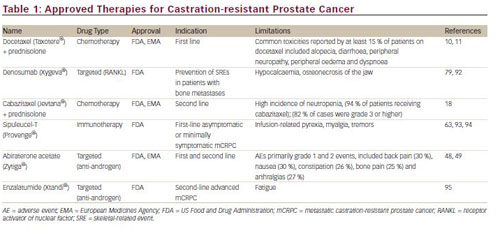
During the past decade, a number of new approaches have been developed for the treatment of CRPC, including hormonal agents, cytotoxic chemotherapeutic drugs, targeted therapeutics and immunotherapy. These developments have led to the regulatory approval of five new therapeutic agents: sipuleucel-T, denosumab, abiraterone acetate, cabazitaxel and enzalatumide (see Table 1). This article will review recent advances in chemotherapy and discuss the potential molecular targets for new therapeutic approaches.
Chemotherapy
Historically, prostate cancer was considered refractory to cytotoxic chemotherapy,9 and chemotherapy options for the treatment of CRPC were limited. Until 2004, only mitoxantrone was approved, providing palliation but no survival benefit. In 2004, docetaxel received approval from the US Food and Drug Administration (FDA) after clinical studies showed that prednisone and docetaxel showed an overall survival (OS) benefit compared with mitoxantrone plus prednisone, as well as a significant improvement of quality of life and pain reduction.10,11
Docetaxel and prednisone in combination are currently considered the standard of care for men with CRPC with detectable metastatic disease.12 However, survival rates remain low: less than 2 years13,14 and less than a year in metastatic cases.15 Moreover, a significant proportion of men with CRPC do not respond to docetaxel-based therapy, and all patients will ultimately develop resistance.16 Mitoxantrone has been the standard treatment following docetaxel failure.17 New chemotherapeutic agents have recently been evaluated for CRPC. Cabazitaxel was approved by the FDA in 2010 for second-line use in CRPC following docetaxel-based treatment. The approval was based on results of the phase III TROPIC trial in which it improved OS in patients whose disease had progressed during or after docetaxel-based therapy.18
Other new chemotherapeutic agents include a novel class of tubulepolymerising agents – epothilones. One agent in this class, ixabepilone, has demonstrated activity in phase II trials in patients with chemotherapynaïve metastatic CRPC19 and as second-line chemotherapy.20 Satraplatin, an oral platinum analogue, was not approved by the FDA as a result of significant toxicities.21
The role of chemotherapy in CRPC has evolved over the last decade. However, to date, docetaxel remains the only first-line chemotherapy option that improves survival, and treatment with docetaxel inevitably results in resistance. Targeting the processes responsible for resistance presents an alternative therapeutic strategy for CPRC.
Potential Molecular Targets for New Therapeutic Approaches to Castration-resistant Prostate Cancer
The advent of molecular-based therapy has resulted in a large number of new therapeutic approaches to CRPC. Potential molecular targets include hormonal and non-hormonal intracellular pathways, such as the apoptotic pathway, signal transduction pathways, angiogenesis, and other oncogenic survival pathways (see Figure 1).
Androgen Receptor Pathway
There is evidence to suggest that although ADT removes the gonadal testosterone in CRPC, androgens originating from other sources, including the adrenal gland and the tumour itself, may continue to act as a ligand and result in AR signalling.22,23 Therefore the AR pathway represents a therapeutic target for CRPC. Established antiandrogen therapy includes AR antagonists, such as bicalutamide and flutamide, and antiandrogenic drugs, such as ketoconolazone, but these result in increased levels of AR, which confer resistance.24 This has led to the development of new antiandrogens. In addition to AR, novel therapies have targeted cytochrome P450 c17 (CYP17) an enzyme involved in androgen synthesis.25 Conversion of testosterone to the more potent dihydrotestosterone (DHT) by 5-α-reductase in tumour tissue also results in continued AR activation, and provides another therapeutic target.26
Apoptosis
Non-hormonal strategies for the treatment of CRPC include inducing stress responses that target apoptosis. Antiapoptotic stress-induced factors include heat shock proteins and chaperone proteins. Elevated levels of the stress-activated cytoprotective chaperone protein clusterin (CLU) have been observed in several cancers, including prostate cancer.27,28 CLU is upregulated in response to many anticancer therapies and confers broad-spectrum resistance by inhibiting protein aggregation and proteotoxic stress, cytochrome C release and activation of apoptotic proteins.29 Expression of CLU in prostate tumours increases after treatment with ADT30 or chemotherapy.7
Survivin antagonists also act through the apoptotic pathway. Survivin inhibits apoptosis, enhances cell proliferation and promotes tumour angiogenesis in a number of tumour types including prostate cancer.31 It is upregulated in malignant tissue and its suppression leads to tumour growth, making it a potentially important therapeutic target.
Insulin-like growth factor receptor-1 (IGF-1R) also has antiapoptotic activity; its other mechanisms of action involve mitogenesis and cellular transformation. Several studies have suggested that it may be important in prostate carcinogenesis and is often overexpressed in prostate tumours.32,33
Signal Transduction Pathway Inhibitors
Upregulation of the phosphatidylinositol 3-kinase (PI3K/AKT) pathway is important in the pathogenesis in prostate carcinogenesis.34 A critical component of this oncogenic survival pathway is activation of the mammalian target of rapamycin (mTOR ) pathway, providing a rationale for the use of mTOR inhibitors in CRPC. P13K activation is regulated by phosphatase and tensin homologue (PTEN). Recent findings suggest that the PTEN tumour suppressor gene may be a useful predictive biomarker in this setting.35
Angiogenesis
Angiogenesis is thought to have an important role in the progression of prostate cancer and provides several potential therapeutic targets in CRPC. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) play a major role in promoting angiogenesis and tumour progression in a number of tumour types. Plasma VEGF levels are significantly elevated in patients with CRPC compared with patients with localised disease.36 Hepatocyte growth factor also promotes angiogenesis. Overexpression of hepatocyte growth factor and its receptor protein, c-MET, has been observed in prostate cancer,37 and androgen suppression has been shown to increase MET expression.38
The Bone Microenvironment
The bone is an important target in advanced CRPC since most patients will develop bone metastases during the course of their disease, and most disease-related symptoms are directly related to bone metastases The nuclear factor κB ligand (RANKL) is involved in the regulation of bone metabolism and is overexpressed in osteoblasts.39Targeting of the bone microenvironment can delay bone metastasis in men with prostate cancer.
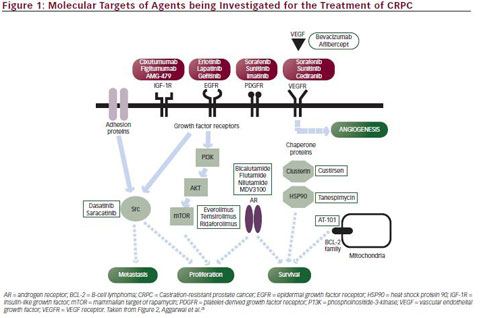
Src kinase signalling is another potential molecular target in CRPC. Src kinases are non-receptor tyrosine kinases that affect several molecular pathways involved in neuropeptide-induced prostate cancer growth and migration, including angiogenesis, survival and transition to CRPC.40 Overexpression of Src and Src kinases has been observed during prostate tumour growth and metastasis, and high Src kinase activity has been associated with shorter OS.41 Src signalling is also involved in the development of bone metastasis, as it regulates different osteoclast functions including bone resorption.42
Endothelin-1 (ET-1), part of the endothelin family of peptides, promotes prostate cancer progression by a number of mechanisms, including angiogenesis, and may predict OS in CRPC.43 It also has an important role in the pathogenesis of bone metastases: ET-1 produced by metastatic cancer cells stimulates the endothelin-A receptor in osteoblastic cells.44
Immune Targets
The field of cancer immunotherapy is rapidly evolving. One promising approach involves augmentation of cell-mediated immunity by interrupting T-cell pathways responsible for immune down-regulation or tolerance. This has provided a number of potential therapeutic targets, including the signalling molecule cytotoxic T-lymphocyte antigen 4 (CTLA-4).45
Epigenetic Regulation of Androgen Receptor Gene Expression
Epigenetic regulation of AR gene expression may also present novel molecular targets in CRCP therapy. Regulation mechanisms include DNA methylation and histone deacetylation.46
Targeted Agents Recently Approved or in Clinical Development
Numerous targeted agents are currently in clinical development; published studies of phase II and III clinical trials are summarised in Table 2. Clinical endpoints include progression-free survival (PFS), OS and levels of PSA, an indicator of disease progression.47 Recently developed antiandrogen therapies target androgen production from both endocrine and autocrine sources. Abiraterone acetate is a selective, irreversible inhibitor of CYP17 and has been approved by the FDA for first- and second-line therapy in the treatment of CRPC following the results of two phase III clinical trials.48,49 Other CYP17 inhibitors in clinical development include TA K-700 (orteronel).50
Enzalutamide (MDV3100), an AR antagonist, significantly improved OS in men with CRPC following docetaxel treatment in a recent phase III clinical trial and received FDA approval in 2012 for the treatment of patients with mCRPC who have previously received docetaxel.51 Dutasteride, a 5α-reductase inhibitor that is used in benign prostatic hypertrophy, has shown only modest efficacy in phase II trials.52,53
Among the numerous targeted therapies in clinical development for CRPC, custirsen is one of the most promising. Custirsen is an intravenously administered antisense oligonucleotide that has demonstrated high affinity for CLU messenger RNA (mRNA) and decreases CLU expression.54,55 In preclinical studies, treatment with custirsen enhanced the effects of cytotoxic drugs, including docetaxel and mitoxantrone.55,56 In a phase II clinical trial, treatment with custirsen plus docetaxel and prednisolone was well-tolerated and associated with a significant improvement in OS.57 In another phase II clinical trial, combined therapy of custirsen plus either docetaxel retreatment (DPC) or mitoxantrone (MPC) was evaluated in patients with progressive mCRPC following first-line docetaxel therapy.58 The results showed improved OS and time to pain progression in the DPC arm compared with the MPC arm. Ongoing phase III trials, one with a primary endpoint of survival and one with an endpoint of pain palliation, are evaluating the addition of custirsen to second-line cabazitaxel/prednisone treatment (AFFINITY trial)59 and to docetaxel/ prednisone in the first-line setting (SYNERGY trial).60
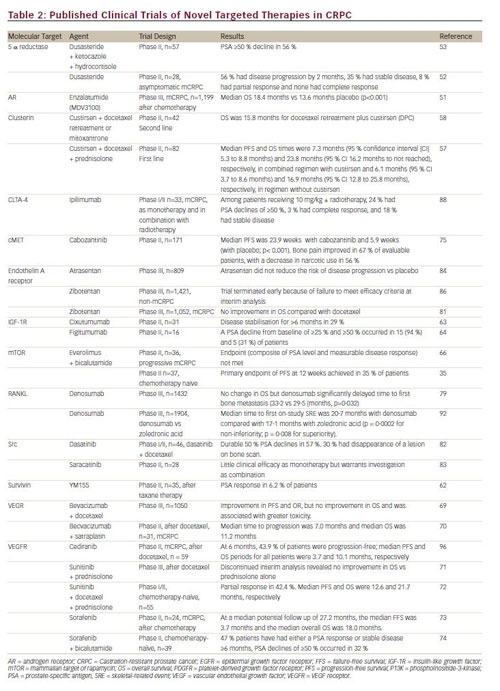
In addition to custirsen, other agents that target the apoptotic pathway are currently being developed. These include two survivin antagonists: LY -218130, an antisense oligonucleotide that complementarily binds to survivin mRNA and inhibits its expression in tumour tissue,61 and YM-155, a small molecule surviving inhibitor. YM-155 has shown modest clinical efficacy in a phase II trial62 and is being investigated in combination with docetaxel. Three human monoclonal antibodies that specifically target IGF-1R are also in clinical development. Cixutumumab63 and figitumumab64 have shown clinical efficacy in phase II trials. Ganitumab (AMG-479) is in the early stages of development.65
The importance of the PI3K/AKT pathway has led to investigation of the use of mTOR inhibitors in CRPC. However, a recent phase II study failed to show significant clinical benefit for the combination of everolimus and bicalutamide in men with CRPC.66 A more recent phase II study has suggested that the PTEN tumour suppressor gene may be a predictive biomarker for response.35 Temsirolimus is also being evaluated, and appears to demonstrate activity in chemotherapy-naïve patients with CRPC.67 A novel mTOR inhibitor, ridaforlolimus,68 and an oral pan-PI3K inhibitor, BKM-120, are also in clinical development.34
Despite a strong rationale for the use of anti-angiogenic strategies in CRCP, a phase III clinical study failed to show a survival advantage for the VEGF inhibitor bevacizumab in combination with docetaxel compared with docetaxel alone.69 However, the combination of bevacizumab and satraplatin showed efficacy in a phase II trial of mCRPC patients.70 Tyrosine kinase inhibitors have also failed to show significant clinical benefit to date. A phase III trial of sunitinib was terminated early when interim analysis revealed no significant improvement in OS.71 In a recent Pphase II trial, a combined treatment regimen involving sunitinib and docetaxel showed more promising clinical activity.72 Sorefanib showed limited clinical activity as monotherapy in a phase II trial,73 although a more recent phase II trial of sorefanib in combination with bicalutamide showed encouraging results.74
Cabozantinib, a tyrosine kinase inhibitor with activity against MET and VEGFR 2, has demonstrated efficacy in CRPC in a recent phase II trial.75 Another angiogenesis-inhibiting agent, tasquinimod, has slowed progression and improved PFS in a phase II study of CRPC,76 though its exact mechanism of action is not clear. Two phase III trials of cabozantinib, COMET-1 and COMET-2, are underway.77,78
Denosumab, a fully human anti-RANKL monoclonal antibody, was approved by the FDA in 2010 for the prevention of skeletal-related events (SREs) in patients with bone metastases from CRPC. In a recent phase III trial, despite showing no improvement in OS, it significantly delayed time to first bone metastasis.79 However, the FDA rejected an application for approval for the use of denosumab in this indication; the effect on bone metastasis-free survival was considered of insufficient magnitude to outweigh the risks of the treatment, which include osteonecrosis of the jaw.80 In another phase III trial, denosumab demonstrated superiority over zoledronic acid for the prevention of SREs.81
Several Src kinases are in clinical development. Dasatinib in combination with docetaxel has shown efficacy in a phase I/II clinical trial,82 and is currently being investigated in a phase III trial. Saracatinib showed limited clinical efficacy in a phase II trial.83
Atrasentan, an inhibitor of the endothelin-A receptor, showed promise in phase II trials, but failed to show significant clinical benefit in patients with CRPC in a phase III trial.84 The phase III SWOG 0421 trial of atrasentan plus docetaxel as first-line therapy was terminated early due to failure to meet primary endpoints.85 Another endothelin-A receptor antagonist, zibotentan, is no longer under investigation as a potential treatment for CRPC following two recent phase III trials in which it failed to demonstrate efficacy.81,86 Immune checkpoint inhibitors, such as ipilimumab, have shown significant efficacy in melanoma. As a result, a number of ongoing clinical trials are investigating the role of ipilimumab in CRCP, either alone or in combination with immunomodulating agents, such as radiation and chemotherapy, and in combination with cancer vaccines.87 In a phase I/II trial, ipilimumab in combination with radiotherapy showed clinical efficacy with manageable adverse events.88 Among the epigenetic therapeutic approaches to CRPC, histone deacetylase inhibitors have been the most widely studied. In a phase II trial, vorinostat was associated with significant toxicities.89 Panobinostat in combination with docetaxel has recently completed phase I testing and a phase II trial is ongoing.90 Azacitidine is an inhibitor of DNA methylation and has shown encouraging results in a phase II trial of chemotherapy-naïve CRPC patients.91
Summary and Concluding Remarks
The field of CRPC therapy is rapidly evolving as a result of the large number of therapies in clinical development. However, clinical trials have demonstrated varied efficacy, with several phase III trials being terminated early for futility, most likely owing to the biological heterogeneity of CRPC. Among the new agents for which phase III trial data are available, the chemotherapeutic agent cabazitaxel and the targeted therapies abiraterone acetate and enzalatumide have shown improvements in OS. Denosumab has delayed the time to bone metastasis. Of the agents being investigated in Phase III trials, custirsen, cabozantinib and dasatinib appear to be the most promising.
The growing number of approved and experimental therapies in CRCP raises many questions. It is difficult to compare clinical trial data for these therapies because of differences in study designs such as heterogeneous patient populations and control arms. There is a lack of studies directly comparing therapies in order to better evaluate their clinical efficacy. The failure of several clinical trials of targeted therapies for CRCP highlights the need for improved design of clinical trials in order to identify agents that have limited efficacy, while enabling more promising therapies to progress more quickly to the approval process. The mixed clinical trial data also illustrates the fact that CRPC is a heterogeneous condition, and patient subgroups may exist that are characterised by varying degrees of involvement of different signalling pathways. There is a need for improved patient selection based on clinical or molecular characteristics, to identify those most likely to benefit from a particular therapy.
The development of molecular targeted therapy calls into the question the future of traditional chemotherapeutic strategies. However, the need for cytotoxic approaches is likely to remain; their optimum utility will be best achieved in combination with targeted therapies. Considerable research is required to determine to optimum sequencing and combinations of these drugs to overcome resistance to monotherapies.
The advent of targeted therapies has brought new treatment paradigm of CRPC. In the future, identification of different molecular subtypes of CRPC, as well as predictive biomarkers of therapeutic response, will allow clinicians to optimise therapy of CRPC through individualised approaches.















