Targeted alpha therapy (TAT) is the delivery of high-energy alpha particles to cancer cells and the tumour microenvironment to treat cancer. After a survival benefit was shown for radium-223 versus standard of care in patients with metastatic castration-resistant prostate cancer (mCRPC), radium-223 became the first approved TAT.1–3 In the European Society for Medical Oncology (ESMO) guidelines for urogenital cancer, radium-223 is currently recommended for men with bone-predominant, symptomatic mCRPC without visceral metastases.4
Since the development of radium-223, the therapeutic landscape has changed; several other compounds have also shown a survival advantage in patients with mCRPC. Therefore, the current treatment challenge is how best to sequence radium-223 to optimise survival.5,6 In a satellite symposium, an expert panel explored the place of radium-223 in the treatment of mCRPC based on mechanisms of action, clinical trial experience and case presentations in current clinical settings. Since this symposium took place, the ERA-223 study (ClinicalTrials.gov Identifier: NCT02043678; n=806), which was investigating the simultaneous initiation of radium-223 and abiraterone/prednisone, was unblinded based on a recommendation from the Independent Data Monitoring Committee. The data from the unblinded study showed a safety signal in the treatment arm with regards to both skeletal fractures and overall survival (OS). Based on this data, the European Medicines Agency (EMA) has issued a label change contraindicating the combination of radium-223 and abiraterone/prednisone.7 Despite these recent findings of an experimental combination, TAT remains a valuable and effective treatment in mCRPC with the potential to improve outcomes in many patients with various other treatment combinations under clinical investigation.
Treatment landscape in metastatic castration-resistant prostate cancer
For many years, docetaxel was the only treatment for mCRPC with a demonstrated survival benefit; however, more recently several compounds have been approved for this indication including abiraterone, enzalutamide, radium-223, sipuleucel-T (US only) and cabazitaxel. The approval of these compounds in mCRPC was based on clinical trials showing modest gains in absolute survival both before and after docetaxel, with consistent hazard ratios (HRs) of the newer compounds ranging between 0.63 and 0.78 depending on how early in the disease course treatment was given.8,9
The second-generation androgen pathway inhibitors abiraterone and enzalutamide were initially approved for patients with symptomatic, progressive mCRPC following docetaxel chemotherapy after demonstrating improved OS versus placebo and standard of care.10–13 Building on this experience, abiraterone and enzalutamide were assessed in asymptomatic or mildly symptomatic metastatic disease, and were shown to improve OS versus standard of care, thus extending the use of both compounds to before docetaxel in mCRPC.14,15 At the same time, the approval of radium-223 was supported by clinical data in patients with mCRPC demonstrating an OS benefit and delayed onset of symptomatic skeletal events for patients treated with radium-223 compared with placebo and standard of care.16,17 The survival benefit was observed for radium-223 versus standard of care when given to patients with mCRPC and no visceral metastases either as an initial treatment or second-line after docetaxel chemotherapy. Patients with metastatic prostate cancer with high volume disease may benefit from docetaxel chemotherapy while they are hormone-naive, but in mCRPC docetaxel is often reserved for patients with progressive symptomatic disease after receiving abiraterone/enzalutamide and radium-223 treatment.18–20
Radium-223 – mechanisms of action
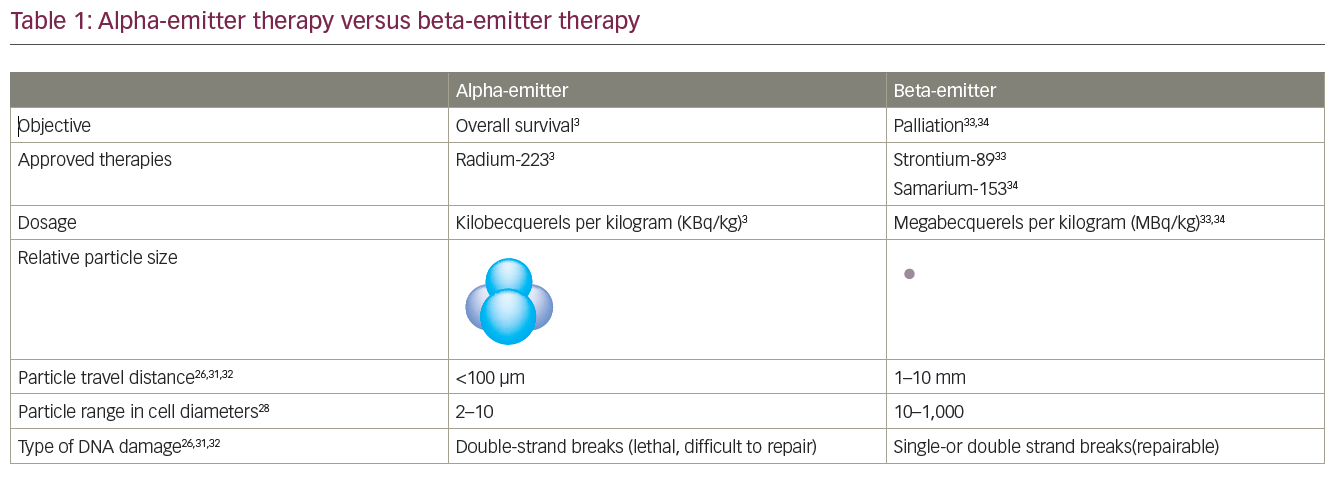
Radium-223 is an example of a mechanism-mediated TAT. This involves the selective targeting of tumour cells in specific organs or tissues based on inherent chemical properties.5,21,22 As a calcium mimetic, the alpha-emitting radioisotope radium-223 is taken up by bone where it selectively binds to areas of increased bone turnover, including bone metastases. It is administered intravenously and travels from the bloodstream to deliver a radioactive ‘payload’ to tumour cells and other cells in the bone microenvironment.2,3,5,6 With radium-223, 95% of energy emission is via cell-killing alpha particles, but the accompanying 1% gamma emission is enough to observe distribution with a gamma camera as demonstrated in clinical trials. Gamma imaging after radium-223, which is not required for everyday practice, shows that a powerful dose of radiation is delivered directly to the sources of bone metastases.2,23–25 Alpha particles form ionising radiation at a short range (50–100 μm), which effectively destroys small clusters of isolated cancer cells, with minimal exposure to healthy tissue.26 Off-target exposure is also minimised as <2% of radium-223 absorbed into hydroxyapatite of bone becomes dislodged.27 The advantages of alpha-emitters over beta-emitters include a shorter range, higher linear energy transfer and the ability to cause double-stranded DNA breaks that result in cell cycle arrest and cell death (Table 1).26,28–35
Tumour growth and bone remodelling in mCRPC involves a vicious cycle of abnormal osteoblastic bone growth in prostate cancer-derived bone metastases, and paracrine factors from prostate cancer cells that affect osteoblasts and osteoclasts.36,37 The resulting abnormal bone remodelling stimulates osteoblasts to secrete factors that promote the growth of bone metastases, which further stimulates osteoclast activity. The mechanisms of action by which radium-223 disrupts this vicious cycle have been demonstrated in a mouse model of osteolytic breast cancer and, more recently, in two mouse xenograft models of advanced prostate cancer.29,30 In the mouse xenograft models, radium-223 inhibited tumour-induced osteoblastic bone growth, protected normal bone architecture and reduced bone volume.29 In tumour-bearing tibiae, double-stranded DNA breaks increased in tumour cells at 24 and 72 hours after one injection of radium-223 compared with the vehicle control, and double-stranded DNA breaks were increased in osteoblasts and osteoclasts at 72 hours.29
Potential synergies between the mechanisms of action of radium-223 and those of various other anti-cancer agents are of particular interest. These could conceivably create highly effective treatment combinations in prostate and other cancer types.38
Radium-223 – pivotal trial data
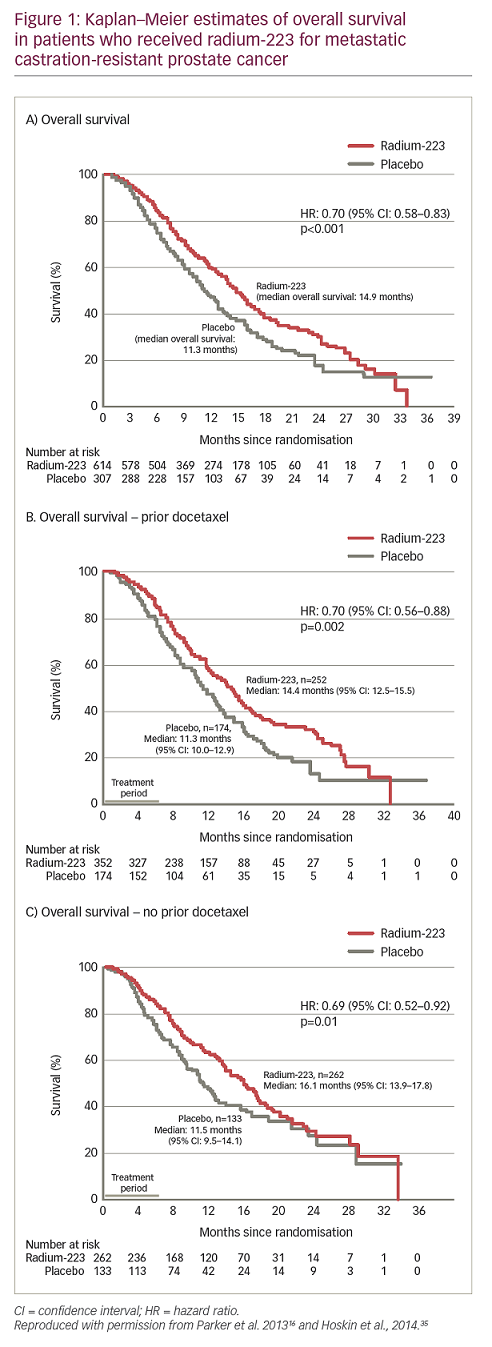
The approval of radium-223 in the US and EU in 2013 was based on the pivotal ALpharadin in SYMPtomatic Prostate Cancer (ALSYMPCA) trial (n=921), which demonstrated an OS benefit compared with the standard of care in patients with bone predominant mCRPC.16,17 In this trial, patients with mCRPC received radium-223 before or after docetaxel, with the choice of initial treatment based on whether a patient was fit enough for chemotherapy. The final results from the trial showed that the risk of death was reduced by 30% for radium-233 compared with standard of care, and the median OS for radium-223 and standard of care was 14.9 months and 11.3 months, respectively – a 30% improvement of 3.6 months (HR: 0.70; p<0.001; Figure 1).16,31–5 In addition, the median time to first symptomatic skeletal event was 15.6 months with radium-223 versus 9.8 months with standard of care (HR: 0.66). Radium-223 also reduced the need for external beam radiation therapy for bone pain (HR: 0.67) and the risk of spinal cord compression (HR: 0.52) compared with standard of care.39
In the ALSYMPCA trial, substantial declines in prostate-specific antigen (PSA) levels were not demonstrated in the radium-223 group, and PSA levels were not a useful measure of the response to treatment.40 However, declines in total alkaline phosphatase (tALP) were observed in 87% of the radium-223 group by week 12, compared with 23% of the standard of care group, and the dynamic profile of tALP was suggestive of a rapid and progressive inhibition of ALP production by osteoblasts. Although tALP levels correlated with OS, the biomarker was not established as a surrogate of disease progression.40
The 3-year safety follow up of the ALSYMPCA trial showed that radium-223 was well tolerated with a low incidence of grade 3 or 4 myelosuppression.41 The haematological treatment-emergent adverse events anaemia, neutropenia and thrombocytopenia were 13%, 2% and 7%, respectively in the radium-223 group, and 13%, 1% and 2%, respectively in the standard of care group. The incidence of myelosuppression remained low, and radium-223 continued to be well tolerated with no new safety signals.41
Contemporary use of radium-223
At the Advanced Prostate Cancer Consensus Conference in March 2017 a panel of about 60 experts was asked their opinion on the optimal method of treating advanced prostate cancer based on the current literature. Panellists with clinical experience of using radium-223 provided consensus regarding the measurement of biomarkers, imaging for staging and treatment monitoring.42
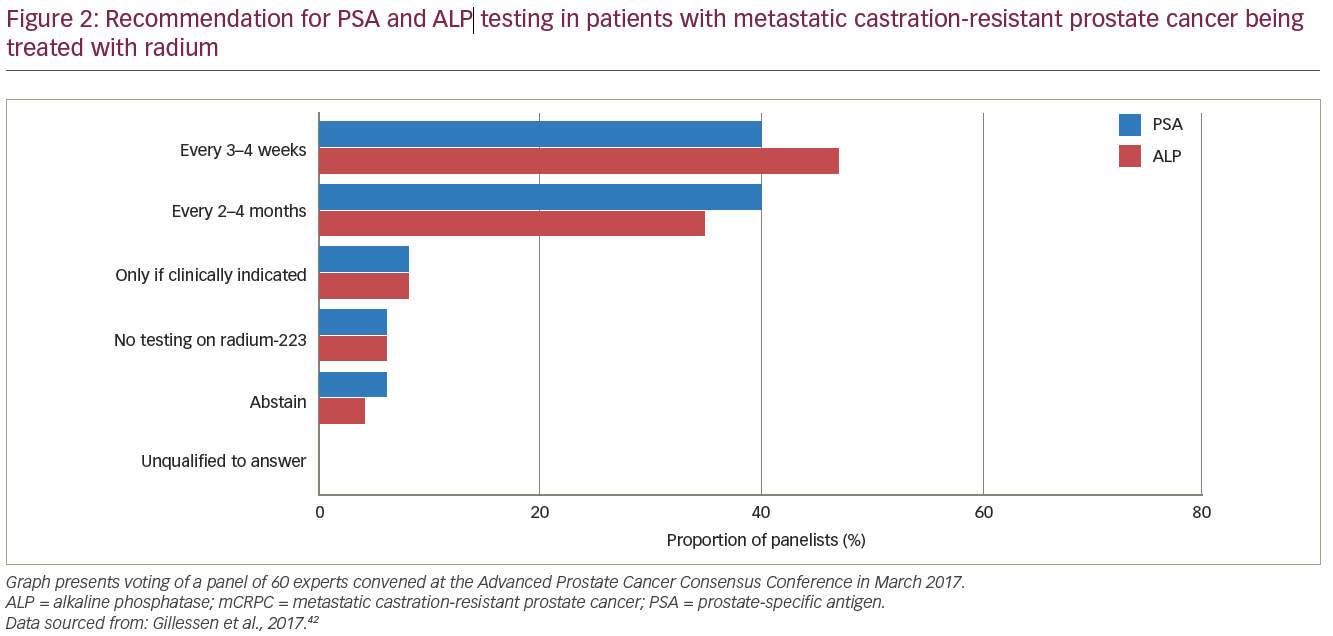
The majority of panellists supported measuring PSA every 3–4 weeks (40%) or every 2–4 months (40%), and measuring tALP every 3–4 weeks (47%) or every 2–4 months (35%) during radium-223 treatment (Figure 2). However, based on the ALSYMPCA trial, it was agreed that there were no biomarkers for response to treatment in mCRPC. When asked about preferred methods of imaging for staging and treatment monitoring during radium-223 treatment, the panel opted for computed tomography (CT) and bone scintigraphy (75%), and next-generation imaging (23%) such as whole-body magnetic resonance imaging (MRI) with diffusion imaging and/or positron emission tomography (PET)/CT with prostate cancer-specific PET radiotracers, choline or fluciclovine. In terms of timing of imaging, panellists chose from the following options: 1) imaging every 3–4 months during and after treatment (41%); 2) 6 months after the completion of radium-223 treatment and every 3–4 months thereafter (27%); or 3) 6 months after the completion of radium-223 treatment and follow-up imaging at clinical or biochemical progression (24%).
The panel was asked for their preferred approach to treating men who are receiving radium-223 for mCRPC and have disease progression outside of the bone but had not previously received a second-generation androgen pathway inhibitor. Of the available options, 52% chose to add abiraterone or enzalutamide, 20% chose to add abiraterone or enzalutamide only in selected patients, and 26% chose to stop treatments including radium-223.42 It should be noted that this voting occurred before the 27 July 2018 label guidance from the European Medicines Agency (EMA)’s Committee for Medicinal Products for Human Use, which considered data from the phase III ERA-223 study (ClinicalTrials.gov Identifier: NCT02043678; n=806).43 Based on this data, the EMA Pharmacovigilance Risk Assessment Committee (PRAC) ruled that in the EU, a combination of radium-223 and abiraterone (Zytiga®, Janssen, Beerse, Belgium) with prednisone/prednisolone should be stopped and a contraindication has been added to the label.7
Case presentations
The use of radium-223 in a contemporary setting has been described in case studies of men at different stages of advanced prostate cancer. In each case described below, favourable biochemical and clinical responses were observed in patients who received radium-223 that was layered on to treatment with a second-generation androgen inhibitor. The treatment approach in these case examples was different than that studied in ERA-223, in which radium-223 and abiraterone plus prednisone were commenced at the same time in all patients who were randomised to receive this combination. The case examples differed as the combination of the two drug classes occurred upon disease progression. Nonetheless, the PRAC recommendation has contraindicated any combination of radium-223 and abiraterone/prednisone.
Abiraterone added to radium-223 in advanced prostate cancer
A 76-year-old male was diagnosed with metastatic advanced prostate cancer and was started on androgen deprivation therapy and denosumab in March 2012; in December 2012, rising PSA levels indicated castration resistance. In January 2013, docetaxel was started resulting in a PSA decline of ≥50% that was sustained for 3 months. In September 2013, the patient developed increasing pelvic pain and had rising PSA levels and was re-staged using a bone and CT scan, and found to have stable disease. MRI of the long-spine excluded significant epidural disease. In October 2013, the patient was started on radium-223 under the expanded access programme.
With each infusion of radium-223, both PSA and tALP increased within the normal ranges. In December 2013, after three doses of radium-223, on CT scan the prostate tumour had increased in size with possible rectal wall infiltration; on bone scan there was possible necrosis of the jaw, which was later confirmed. Abiraterone was layered on to radium-223, which resulted in a rapid decrease in PSA, decreased prostate size and no new bone lesions. The patient continued with abiraterone for 3 years, after which his disease progressed and he received second-line cabazitaxel.
The clinical observations in the case presentation suggest that in men receiving radium-223 CT should be considered after three doses or if soft tissue progression is suspected. PSA and tALP should be monitored in patients receiving radium-223, although they are not markers of disease progression.
Radium-223 layered on after progression on enzalutamide in asymptomatic metastatic castration-resistant prostate cancer
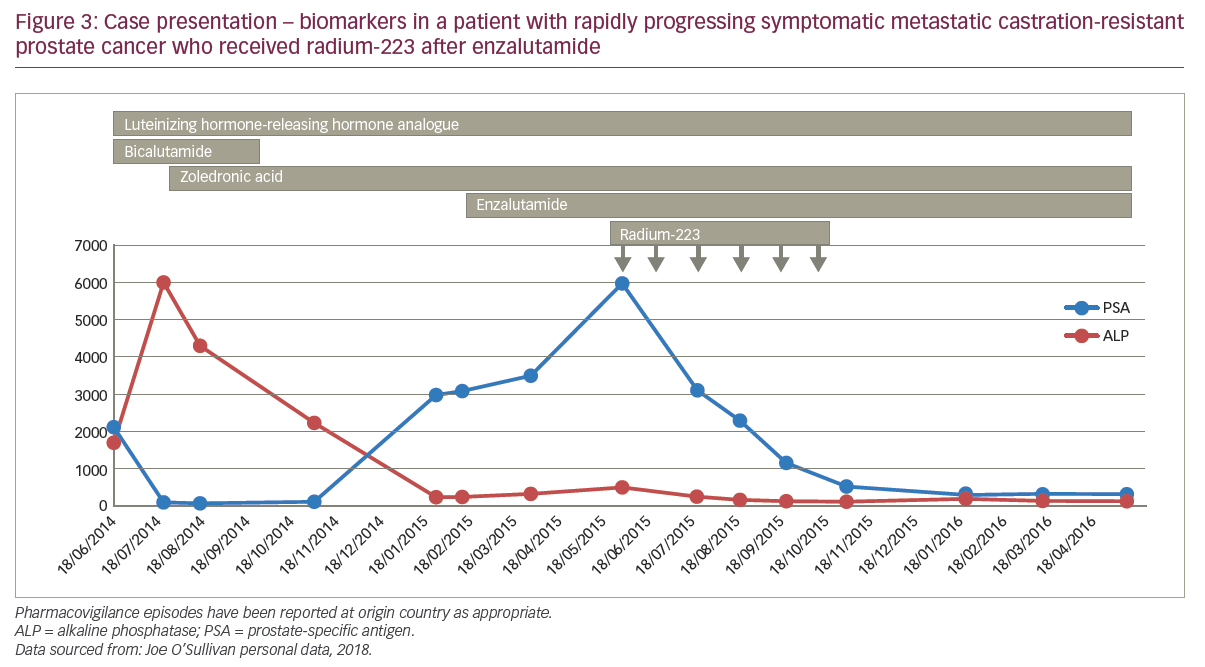
A 61-year-old male presented with T3 prostate cancer and a PSA of 500 ng/ml after responding to androgen deprivation therapy for 9 years. The patient received eight cycles of docetaxel and maintained reasonable control for 4 months, until at 5 months PSA levels started to increase. The patient was re-staged and found to have asymptomatic bone metastases and no visceral involvement. The patient received six cycles of enzalutamide and after an initial period of stability PSA levels increased. Radium-223 and denusomab were layered on to enzalutamide. Within 3 months of starting radium-223, the patient’s PSA levels decreased to 0.1 ng/ml and 18 months later (currently) the patient was still responding to treatment (Figure 3).
Radium-223 layered on to enzalutamide in rapidly progressing symptomatic metastatic castration-resistant prostate cancer
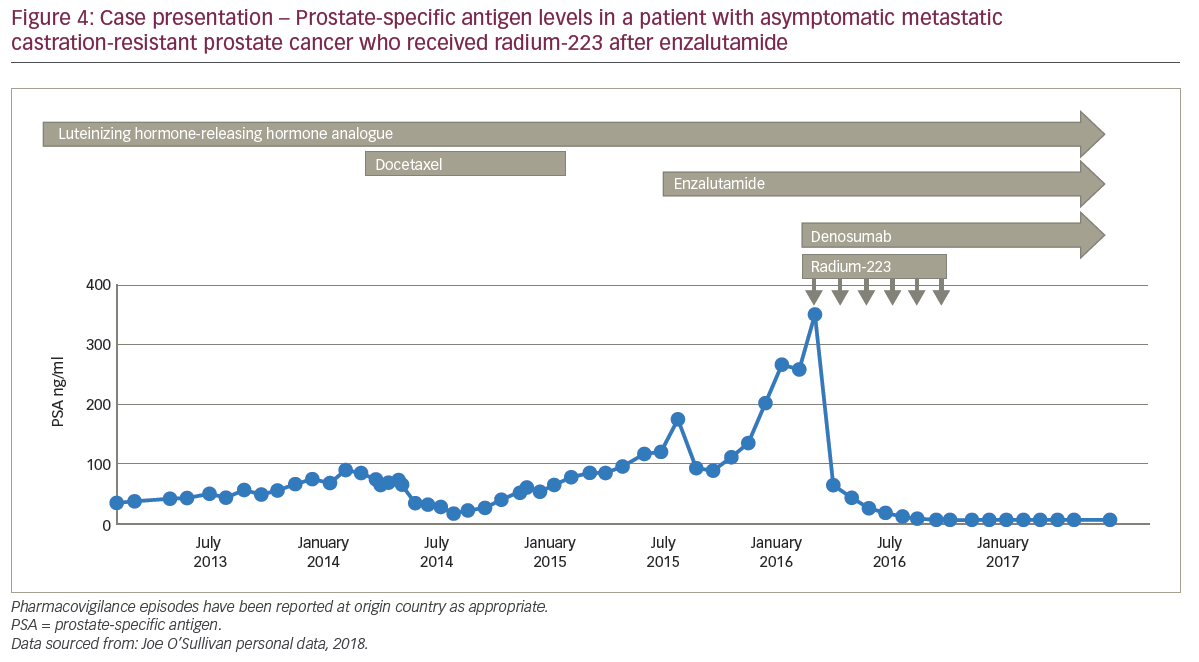
A 67-year-old male was very ill when he presented with nocturia and long-term worsening back pain. His sister had previously died of neutropenic sepsis during chemotherapy for breast cancer. The patient’s bone scan showed diffuse bone involvement and stage T3 prostate cancer with a PSA of 2,000 ng/ml and alkaline phosphatase (ALP) 6,000 U/l. The patient started hormone therapy and his back-pain continued for which analgesia was needed; the patient refused docetaxel. After 3 months of androgen deprivation therapy, the patient’s PSA nadir fell from 2,000 to 50 ng/ml; tALP started to rise rapidly so zoledronic acid was started, to which the tALP levels responded well, decreasing from about 6,000 to 1,000 U/l. After a further 3 months, the patient had advanced high-volume prostate cancer and rising PSA levels, for which he was receiving androgen deprivation therapy and zoledronic acid, and he was continuing to refuse cytotoxic chemotherapy. Enzalutamide was started and external radiation to the pelvis was given to control pain. For 2 months of enzalutamide treatment, pain remained controlled and PSA levels continued to increase above 300 ng/ml with stable tALP, before a rapid progression of biochemical markers by 3 months. The patient then received radium-223 which was layered on to enzalutamide, zoledronic acid and androgen deprivation therapy, and after six cycles, PSA declined to about 100 ng/ml with a good pain response (Figure 4). The patient remained pain free but died 12 months after starting radium-223 after developing rapidly progressive disease.
Developments in targeted alpha therapy
Radium-223 is currently the only TAT that is approved for clinical use in mCRPC. In addition to this indication, it is currently under investigation in breast cancer (phase II [ClinicalTrials.gov Identifiers: NCT02258464, NCT02258451, NCT02366130]) and multiple myeloma (phase I [ClinicalTrials.gov Identifier: NCT02928029]). Radium-223 and other TATs are also under investigation in various combinations and indications (Table 2).
An alternative experimental approach to mCRPC therapy is to give radium-233 and immunotherapy in combination. These different treatments may act synergistically and reverse the immunosuppressive response affecting osteoclasts within bone metastases. In an in vitro study, human prostate, breast and lung carcinoma cell lines were exposed to sub-lethal levels of radium-223, and within 96 hours each tumour type had increased cytotoxic T lymphocyte-mediated lysis of tumour cells; that is, radium-223 did not kill the tumour cells – rather it induced the production of cytotoxic T lymphocytes.44 Lysis of each tumour type by CD8+ cytotoxic T lymphocytes was accompanied by augmented protein expression of major histocompatibility compex I (MHC-I) and other molecules that mediate antigen presentation. These data suggest that radium-223 can induce immunogenic modulation in tumour cells that survive irradiation.44


Further interesting potential combinations with radium-223 include various inhibitors of DNA repair pathways, including poly (ADP-ribose) polymerase (PARP) inhibitors, and drugs that target kinases (e.g. ATR, ATM, DNA-PK). These agents may be potent radiosensitisers, producing synergistic anti-tumour activity with TAT.45,46 At a recent American Association for Cancer Research Annual Meeting, data were presented showing that in an in vivo model mimicking mCRPC (intratibial LNCaP-Luc prostate cancer model), there was a synergistic anti-tumour effect between the ATR kinase inhibitor BAY 1895344 and radium-223. This was demonstrated by substantially lower levels of serum PSA and procollagen I N-terminal peptide when this combination was added compared with BAY 1895344 or radium-223 alone or vehicle (Figure 5).47
The flexibility of targeting mechanisms with some alpha-emitters allows for a potential range of applications across various tumour types using novel delivery methods.48 One such approach is to conjugate alpha-emitters to monoclonal antibodies or antibody fragments that target tumour cells.26 The potential of this strategy has been demonstrated in various studies, including an animal model that used a conjugate of thorium-227 to trastuzumab to specifically target ovarian cancer cells.49 A further in vivo study used thorium-227, which was conjugated to an anti-CD33 antibody, and was shown to bind with high affinity to the sialic acid receptor CD33. This approach provides a means of selectively targeting alpha doses to myeloid cells in the treatment of acute myeloid leukemia.50
An additional novel delivery method is created by conjugating the alpha-emitter to small molecules or peptides to target tumour cells. Ligands, synthetic protein scaffolds (e.g. affibodies) and substrate analogues (e.g. peptides) can be used as targeting agents.26 Other investigational compounds such as actinium-225 are chelated to a peptide that binds to the prostate-specific membrane antigen (PSMA) enzyme on target prostatic cells, thereby delivering alpha particles to the tumour.26,43
A further interesting example of TAT in mCRPC treatment is the use of PSMA-617-targeted actinium-225. This treatment is currently being investigated in mCRPC patients as part of an off-trial compassionate access programme. In this programme, two patients achieved a complete response on imaging, with PSA levels falling from 2,923 ng/ml to <0.1 ng/ml despite widespread metastatic disease.51,52 This suggested that actinium-225–PSMA-617 has the potential to benefit patients with advanced prostate cancer. No haematological toxicity has been reported, but it should be noted that six patients (43%) had grade 1 xerostomia, and two patients (14%) had grade 2 xerostomia.
Overall, these advances in the targeted delivery of radionuclides and the increased availability of alpha-emitters have led to a number of recent clinical trials of TAT in prostate cancer, as well as other cancers such as breast and multiple myeloma. Given the flexibility of targeted delivery methods and the direct cytotoxic effects of alpha-emitters, TAT could potentially change the treatment paradigm in several cancer indications.
Conclusions
The proof-of-concept that TAT improves OS with minimal toxicity in patients with advanced prostate cancer was first established during the clinical development of radium-223. More recently, several compounds from other classes have been approved in mCRPC that have changed the treatment landscape and expanded the possibilities for sequencing and combining treatments with different mechanisms of action. Physician experience from real-world clinical practice suggests that, in certain circumstances, radium-223 and the second-generation androgen pathway inhibitors may have potentially synergistic mechanisms of action. In the case presentations, layering radium-223 onto enzalutamide or abiraterone upon clinical progression (or layering on abiraterone to radium-223) provided a good clinical response in men with advanced prostate cancer. However, the use of these combinations has been the subject of a contraindication issued by the EMA, which recommended against any combination of radium-223 with abiraterone and prednisone.7 It should be noted that in the case examples presented above, radium-223 was layered onto the novel anti-hormonal treatment upon progression, whereas in the ERA-223 study patients were given the combination from the outset, before progression occurred (ClinicalTrials.gov Identifier: NCT02043678)43. It is unknown whether toxicities or potential efficacy arising from layering are different from those arising from combining therapies at initiation of treatment, or if there is a difference between abiraterone plus prednisone and enzalutamide when given in combination. Current clinical trials are not comparing these different treatment regimens, so may not fully resolve this issue. Nevertheless, data from ERA-223 and other ongoing studies, as well as greater experience from real-world clinical practice, should better define the optimal use of radium-233 with other treatments in the management of patients with mCRPC and may improve understanding of its role in managing this increasingly prevalent disease.














