Androgen deprivation therapy (ADT) has been the cornerstone of the treatment of metastatic prostate carcinoma for over 70 years, ever since Huggins and Hodges first treated men with prostate carcinoma with orchidectomy or oestrogen injection in 1941.1 However, the treatment landscape of metastatic hormone-sensitive prostate cancer (mHSPC) has changed rapidly over the past decade, with level 1 data to support first-line treatment with ADT in combination with either docetaxel chemotherapy or an androgen-receptor pathway inhibitor (abiraterone/prednisolone, enzalutamide or apalutamide).2–4 Abiraterone blocks androgen biosynthesis via cytochrome P-450c17 inhibition,5 whereas enzalutamide and apalutamide are direct androgen receptor inhibitors.6,7 Docetaxel chemotherapy was the first agent to be routinely added to ADT; in the trials that led to its approval, median overall survival (OS) was extended by approximately 13–15 months, with median survival times of almost 60 months.8–11 Subsequent trials demonstrated that the addition of androgen-receptor pathway inhibitors (abiraterone, enzalutamide or apalutamide) to ADT also statistically significantly improved progression-free survival (PFS) and OS. In addition, results from the STAMPEDE trial (Systemic therapy in advancing or metastatic prostate cancer: Evaluation of drug efficacy [STAMPEDE]; ClinicalTrials.gov identifier: NCT00268476) support the use of prostate radiotherapy in those with low-volume metastatic disease (Table 1).5–24
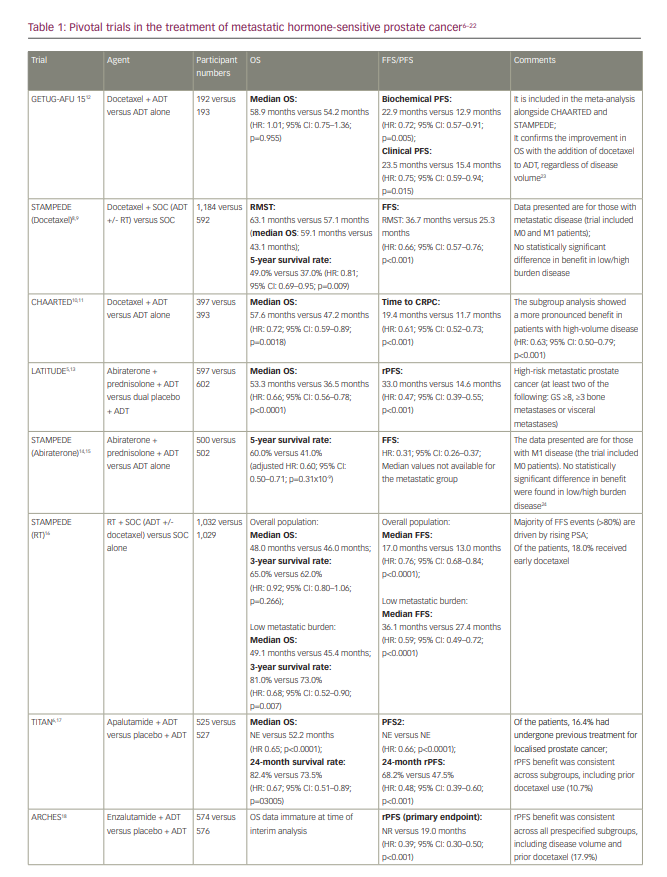
However, no trials have been designed to directly randomize between ADT plus docetaxel and ADT plus an androgen-receptor pathway inhibitor. Within the STAMPEDE trial, the combinations of ADT plus docetaxel and ADT plus abiraterone/prednisolone were each compared with ADT alone, with a period of overlapping randomization, which allowed an opportunistic analysis of comparative outcomes to be conducted. Although not fully powered or a randomized comparison, results were consistent with a potential advantage of the abiraterone combination with respect to failure-free survival (hazard ratio [HR]: 0.51; 95% confidence interval [CI]: 0.39–0.67; p<0.001) but not with respect to OS (HR: 1.16; 95% CI: 0.82–1.65; p=0.40).25,26 A further indirect comparison via a meta-analysis also suggested that the abiraterone combination is associated with a reduced risk of disease progression compared with the docetaxel combination and was at least as effective at reducing the risk of death.27
Many clinicians now favour the addition of an androgen-receptor pathway inhibitor to ADT in the first-line setting for mHSPC, potentially reserving docetaxel for selected patients with high-volume disease, though there is no formal consensus.3 Treatment decisions should be made on an individual patient basis and must take into account whether the disease is de novo or recurrent as well as whether it is ‘high’ or ‘low’ volume. The proportion of each of these characteristics within the relevant clinical trials should be carefully considered when using the results to aid treatment decisions.
Phase III trials are now investigating the potential benefit of triplet combination therapy with ADT, an androgen-receptor pathway inhibitor and docetaxel. This review will consider the results of the recently published PEACE-1 (A phase III study for patients with metastatic hormone-naïve prostate cancer [PEACE1]; ClinicalTrials.gov identifier: NCT01957436) and ARASENS (ODM-201 in addition to standard ADT and docetaxel in metastatic castration sensitive prostate cancer [ARASENS]; ClinicalTrials.gov identifier: NCT02799602) trials and discuss whether they now justify ‘triple therapy’ for mHSPC.
Triplet combination therapy
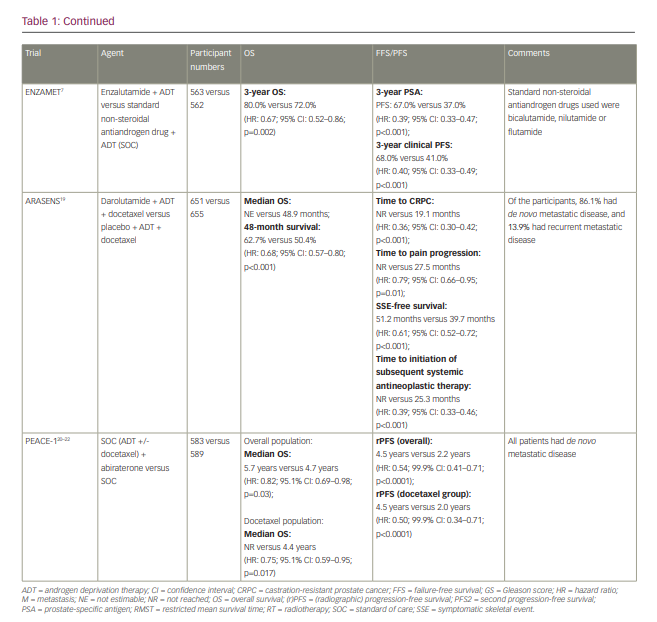
The first available data for triplet therapy came from trials that had been designed primarily to investigate the addition of an androgen-receptor pathway inhibitor to ADT. In the ENZAMET trial (Enzalutamide in first line androgen deprivation therapy for metastatic prostate cancer [ENZAMET]; ClinicalTrials.gov identifier: NCT02446405), which compared ADT plus enzalutamide with ADT plus standard non-steroidal antiandrogen therapy (bicalutamide, nilutamide or flutamide), early docetaxel treatment was planned in 45% of participants after the protocol was amended to allow this following release of the CHAARTED results (Androgen ablation therapy with or without chemotherapy in treating patients with metastatic prostate cancer [CHAARTED]; ClinicalTrials.gov identifier: NCT00309985).7 The effect size of enzalutamide on OS was smaller in those who received early docetaxel (HR: 0.90; 95% CI: 0.62–1.31) than in those who did not (HR: 0.53; 95% CI: 0.37–0.75), though the trial was not designed or powered sufficiently to make this analysis, and it was not subject to randomized comparison.7,10 The TITAN (A study of apalutamide [JNJ-56021927, ARN-509] plus androgen deprivation therapy (ADT) versus ADT in participants with mHSPC [TITAN]; ClinicalTrials.gov identifier: NCT02489318) and ARCHES (A study of enzalutamide plus androgen deprivation therapy [ADT] versus placebo plus ADT in patients with metastatic hormone sensitive prostate cancer [mHSPC] [ARCHES]; ClinicalTrials.gov Identifier: NCT02677896) trials (Table 1), which investigated the addition of apalutamide and enzalutamide, respectively, to ADT, also permitted the use of docetaxel, though both trials required this to be completed prior to the initiation of the study treatment. In both trials, the outcomes for radiographic PFS (rPFS) did not significantly differ according to prior docetaxel use.6,18
PEACE-1 (ClinicalTrials.gov identifier: NCT01957436) was a phase III trial with a 2×2 factorial design conducted in men with de novo mHSPC that set out to compare standard of care (SOC), SOC plus abiraterone, SOC plus radiotherapy (to the primary tumour) and SOC plus abiraterone plus radiotherapy.20–22 When the trial commenced recruitment in 2013, the SOC was initially ADT alone. However, SOC treatment for men with mHSPC rapidly evolved over the course of trial accrual to potentially include docetaxel, androgen-receptor pathway inhibitors and local radiotherapy in those with low-volume disease. As a result, the trial was amended, first in 2015 to permit docetaxel as part of the SOC and then in 2017 to make it mandatory.22 No interaction was detected between the effect of abiraterone and radiotherapy on rPFS, which allowed, according to the statistical design of the study, for the two abiraterone arms to be pooled for analysis. Docetaxel was part of the SOC for 355 (61%) participants in the SOC (+/- radiotherapy) plus abiraterone group and 355 (60%) participants in the SOC (+/- radiotherapy) without abiraterone group. In those receiving docetaxel, the proportion of patients with high-volume metastatic burden was 63% and 65% for patients receiving abiraterone and for those who did not, respectively.
High-volume metastatic burden was defined as per the CHAARTED study as the presence of visceral metastases or at least four bone lesions, with one beyond the vertebral bodies and pelvis.10 In the docetaxel sub-population, after a median follow-up of 3.5 years, the co-primary endpoint of rPFS was 4.5 years and 2.0 years in the SOC plus abiraterone and SOC without abiraterone arms, respectively (HR: 0.50; 99.9% CI: 0.34–0.71; p<0.0001) and was consistent with the result for the trial overall (Table 1). For those with high-volume metastatic burden within the docetaxel sub-population, rPFS was 4.1 years in the SOC plus abiraterone arm versus 1.6 years in the SOC without abiraterone arm (HR: 0.47; 99.9% CI: 0.30–0.72; p<0.0001), whereas for those with low-volume metastatic burden rPFS was ‘not reached’ versus 2.7 years, respectively (HR: 0.58; 99.9% CI: 0.29–1.15; p=0.0061). Within the docetaxel sub-population, after a median follow-up of 3.8 years, there was also a statistically significant improvement in OS with the addition of abiraterone (not reached versus 4.4 years; HR: 0.75; 95.1% CI: 0.59–0.95; p=0.017), which remained significant for those with high-volume metastatic burden (5.1 years versus 3.5 years; HR: 0.72; 95.1% CI: 0.55–0.95; p=0.019); moreover, this improvement occurred despite 84% of participants in the control group receiving at least one life-prolonging treatment beyond progression. Again, this was consistent with the OS outcome for the trial overall (Table 1). Data for OS in those with low-volume metastatic burden are not yet mature. In the ADT with the docetaxel sub-population, grade 3 or higher adverse events occurred in 63% (217/347) of those receiving abiraterone and 52% (181/350) of those not receiving abiraterone. As expected, there was a higher incidence of grade ≥3 hypertension (22% versus 13%), liver dysfunction (6% versus 1 %) and hypokalaemia (3% versus 0%) in those receiving abiraterone, though toxicity was otherwise similar between groups. Notably, there was no increase in haematological toxicity with the addition of abiraterone to ADT plus docetaxel.20–22
ARASENS (ClinicalTrials.gov identifier: NCT02799602), a recently published randomized, double-blind, placebo-controlled phase III trial, evaluated the effect of the addition of darolutamide or placebo to ADT plus docetaxel on OS in 1,306 men with mHSPC.19 Darolutamide is a structurally distinct potent androgen receptor inhibitor. In PEACE-1, all participants had de novo mHSPC, whereas in ARASENS 86.1% had de novo mHSPC and 13.9% had recurrent mHSPC. With a median follow-up of 43.7 months, the primary endpoint of OS was not estimable in the darolutamide arm and was 48.9 months in the placebo arm (HR: 0.68; 95% CI: 0.57–0.80; p<0.001). The rate of 48-month survival was 62.7% and 50.4% in the darolutamide and the placebo arm, respectively. The OS benefit was consistent across most subgroups considered, including de novo versus recurrent disease. The subgroup analysis according to disease volume has not been presented, though it would be of interest for the interpretation of this trial. Darolutamide also resulted in a statistically significant improvement in the secondary endpoints of time to castration-resistant prostate cancer (not reached versus 19.1 months; HR: 0.36; 95% CI: 0.30–0.42; p<0.001), time to pain progression (not reached versus 27.5 months; HR: 0.79; 95% CI: 0.66–0.95; p=0.01), symptomatic skeletal event-free survival (51.2 months versus 39.7 months; HR: 0.61; 95% CI: 0.52–0.72; p<0.001), time to first symptomatic skeletal event (not reached in either arm; HR: 0.71; 95% CI: 0.54–0.94; p=0.02) and time to initiation of subsequent systemic antineoplastic therapy (not reached versus 25.3 months; HR: 0.39; 95% CI: 0.33–0.46; p<0.001). Toxicity was similar in both arms, with an incidence of grade 3–4 adverse events of 66.1% in the darolutamide arm and 63.5% in the placebo arm, and a rate of serious adverse events of 44.8% and 42.3% for the darolutamide and the placebo arm, respectively. The incidence of adverse events, with particular relevance to those receiving androgen-receptor pathway inhibitors, was generally similar across both arms, with the exception of hypertension (13.7% versus 9.2%) and rash (16.6% versus 13.5%).19
Discussion
The PEACE-1 and ARASENS trials have both shown an OS and PFS benefit from the addition of an androgen-receptor pathway inhibitor to the SOC option of ADT plus docetaxel in de novo mHSPC.19–22 Results from PEACE-1 show a larger OS and rPFS benefit in those with high-volume metastatic burden. OS data for those with low-volume metastatic burden are immature, though rPFS data indicate benefit from the addition of abiraterone in this group. Based on these results, it seems clear that, if a clinician decides to treat a patient with mHSPC with the SOC option of ADT plus docetaxel, then an androgen-receptor pathway inhibitor should now be added concomitantly. Patients treated with ADT plus docetaxel, in preference to ADT plus an androgen-receptor pathway inhibitor, are most likely to be those with high-volume disease. A longer-term follow-up of PEACE-1 will also allow for the assessment of whether there is any benefit from the addition of local radiotherapy to triplet combination therapy in this setting.
A key challenge in the interpretation of data for triple therapy, however, is that many clinicians now prefer to use ADT plus an androgen-receptor pathway inhibitor as their SOC first-line treatment for mHSPC, in preference to ADT plus docetaxel, particularly in patients with low-volume disease.19 However, the benefit gained from the addition of docetaxel to this version of SOC combination therapy has not been addressed in a prospective randomized trial, meaning that it is not possible to make definitive recommendations about the addition of docetaxel in this setting. In particular, the likely additional toxicity from adding docetaxel to ADT plus an androgen-receptor pathway inhibitor needs to be carefully considered.
The benefit of the triplet combination treatment in patients with better prognosis disease, such as those with recurrent metastatic disease or node-only disease, is unclear. In ARASENS, only 86 participants (13.2%) in the darolutamide arm and 82 participants (12.5%) in the placebo arm had recurrent metastatic disease. In this cohort, the HR was 0.61 (95% CI: 0.35–1.05) in favour of the darolutamide arm. The proportion of patients with node-only disease at screening was 3.5% and 2.4% in the darolutamide and the placebo arm, respectively; therefore, no meaningful conclusions can be made about the benefit of darolutamide in this group.19 PEACE-1 only recruited patients with de novo metastatic disease with bone or visceral metastases; therefore, the results cannot be applied to those with node-only metastases or recurrent disease.
Importantly, the additional toxicity associated with triplet combination therapy is modest and as may be expected from the known toxicity profile of androgen-receptor pathway inhibitors. For those in whom ADT plus docetaxel is being used as the SOC treatment of choice, this additional toxicity is likely to be acceptable given the survival benefits. However, treatment decisions should be made on an individual patient basis, taking into account patient preference, disease burden, toxicity profiles and access to treatments. PEACE-1 and ARASENS did not address the question of whether triplet therapy is more efficacious than ADT plus an androgen-receptor pathway inhibitor, and further prospective trials are needed before any recommendations can be made about the use of triplet therapy in this group. It is important to note that real-world data show that adoption of doublet therapy remains significantly lower than might be anticipated, despite clear survival outcomes, and it is therefore unclear how quickly triplet therapy will be incorporated into clinical practice.28
These results come in an era of intense interest in the personalized treatment for mHSPC. A series of ongoing trials are looking at the addition of molecularly targeted agents to the combination of an androgen-receptor pathway inhibitor plus ADT. In homologous recombination repair gene-mutated mHSPC, the AMPLITUDE trial (A study of niraparib in combination with abiraterone acetate and prednisone versus abiraterone acetate and prednisone for the treatment of participants with deleterious germline or somatic homologous recombination repair [HRR] gene-mutated metastatic castration-sensitive prostate cancer [mCSPC] [AMPLITUDE]; ClinicalTrials.gov identifier: NCT04497844) is investigating the addition of the poly ADP ribose polymerase inhibitor niraparib to abiraterone plus ADT, whilst TALAPRO-3 (Study of talazoparib with enzalutamide in men with DDR gene mutated mCSPC; ClinicalTrials.gov identifier: NCT04821622) is investigating the addition of talazoparib to enzalutamide plus ADT.29,30 In those with PTEN loss, CAPItello-281 (Capivasertib+abiraterone as treatment for patients with metastatic hormone-sensitive prostate cancer and PTEN deficiency [CAPItello-281]; ClinicalTrials.gov identifier: NCT04493853) is investigating the addition of the AKT inhibitor capivasertib to abiraterone plus ADT.31 Furthermore, for those with oligometastatic HSPC, there is increasing interest in metastasis-directed therapy, with several ongoing clinical trials looking at the role of treatments such as stereotactic radiotherapy and radical prostatectomy.32 If the results of these trials allow us to move towards a personalized approach for mHSPC, then the value of triplet combination therapy for ‘all comers’ may need to be re-evaluated within molecularly or clinically defined subsets.














