The American Cancer Society estimates that approximately 248,530 new cases of prostate cancer will be diagnosed in the United States in 2021.1 For the nearly 90% of patients diagnosed with locoregional prostate cancer, the goal of treatment by surgical intervention or radiation is cure. In some cases, active surveillance is appropriate. For locoregional prostate cancers, these patients have a 100% 5-year survival rate irrespective of initial management strategy.2 Within 10 years of treatment, 20–50% of patients who undergo definitive therapy will develop biochemically recurrent disease, and the likelihood of relapse increases with more aggressive Gleason grades.3,4 Starting intermittent or continuous androgen-deprivation therapy (ADT) is common practice in patients who have biochemical relapse, or in those with higher-risk locoregional disease.5,6 The durability of prostate-specific antigen (PSA) response to ADT is approximately 33.2 months in the non-metastatic castration-sensitive setting.7,8 This represents the natural disease course where the PSA will begin to rise, despite a castration level of testosterone (<50 ng/dL), mostly driven by changes to androgen receptors in the prostate cancer cells.9 At this point, imaging studies subclassify castrate-resistant disease into non-metastatic castrate-resistant prostate cancer (nmCRPC or M0 CRPC) or metastatic castrate-resistant prostate cancer (mCRPC). Traditionally, computed tomography (CT) scans and nuclear medicine bone scans have been used to determine if a patient has metastatic disease, but newer, more sensitive imaging is finding metastatic disease at lower PSA levels, and it is expected that the number of patients with nmCRPC will shrink with increased use of the newer imaging. In 2020, it is estimated that 60,000 men were diagnosed with nmCRPC, and of all patients with nmCRPC 34% had disease that progressed to metastatic disease.10
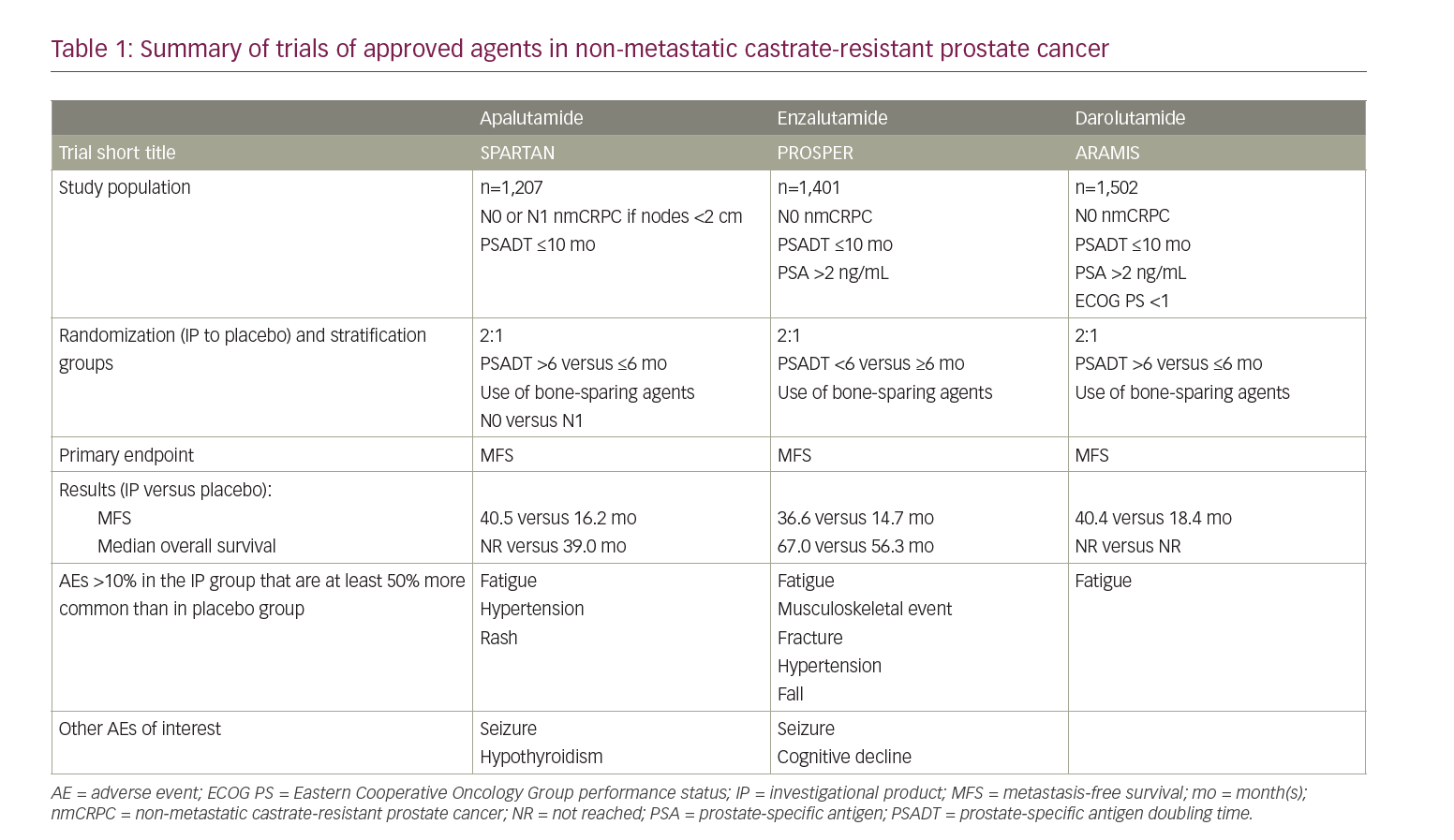
In nmCRPC, the foremost objective of treatment is to prolong the time to metastatic disease with the hope of increasing overall survival. In 2005, an analysis of the placebo arm of a prematurely terminated study identified that only about one-third of patients with nmCRPC developed bone metastases at 2 years, and that baseline PSA and PSA velocity were important predictors of time to progressive disease.11–13 When stratifying PSA velocity, patients with a PSA doubling time (PSADT) of less than 10 months had the highest risk of developing distant metastases.14 The current National Comprehensive Cancer Network guidelines for prostate cancer suggest using this metric as a decision point between observation (with continued ADT) or the addition of next-generation androgen antagonist therapy to ongoing ADT. For those with a PSADT ≤10 months, the guidelines recommend the preferential use of one of three oral next-generation androgen receptor (AR) antagonists that have been approved for nmCRPC: apalutamide, darolutamide and enzalutamide.5 These non-steroidal drugs work similarly; they bind the ligand-binding domain of the AR to prevent AR translocation to the nucleus, DNA binding and AR-mediated transcription. Additionally, compared with the prior generation of AR antagonists, these molecules were noted to have a higher affinity for the AR.15–17 All three next-generation AR antagonists have been shown in large, randomized, double-blind, placebo-controlled trials to increase median metastasis-free survival (MFS) by approximately 2 years. Additionally, follow-up analyses of the same studies showed an improvement in overall survival (OS) in nmCRPC when compared to placebo (Table 1).11,13,18
Apalutamide in non-metastatic castrate-resistant prostate cancer
Apalutamide was the first of the three next-generation AR antagonists to be tested in the setting of nmCRPC.11 In late 2013, the double-blind, phase III Study of apalutamide (ARN-509) in men with non-metastatic castration-resistant prostate cancer (SPARTAN) registry trial started enrolling patients to test the efficacy of apalutamide (240 mg daily) relative to placebo in patients with nmCRPC continued on ADT. These patients had N0 or N1 (allowing for pelvic lymph nodes that measured <2 cm in the short axis below the aortic bifurcation) nmCRPC with a PSADT of 10 months or less. Notably, this study excluded patients with a risk of seizure by screening them for use of medications that lower the seizure threshold and recent neurological events (e.g. stroke), and had them undergo a CT scan of the brain. By the end of the enrolment period, 1,207 men were randomized in a 2:1 ratio to receive 240 mg of apalutamide daily or placebo. During the treatment period, patients completed health-related quality of life (HRQoL) questionnaires, their disease was monitored by conventional imaging at a minimum of 16-week intervals and assessed by blinded independent central review, and both patients and providers were blinded to PSA values during the blinded phase of treatment. After meeting the primary endpoint of 378 metastasis or death events, the trial was unblinded and analysed to reveal a 70% lower risk of metastasis or death in the apalutamide group than the placebo group; an effect that was persistent across age, PSADT and nodal disease subgroups. This translated to a median MFS that was 24.3 months higher in the apalutamide group than the placebo group (40.5 versus 16.2 months).11
The safety profile of the apalutamide regimen included an increased rate of rash, fatigue, arthralgia, weight loss, falls and fractures when compared to placebo. Most adverse events (AEs) were low grade when graded by the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0, and discontinuation owing to an AE was <11% in both groups. HRQoL results showed that patients on apalutamide and those on placebo maintained stable overall health. Of note, when stratified by subgroups, the most notable MFS hazard ratio (HR) ranges were age (HR 0.14 for those <65 years old, 0.25 for those 65 to <75 years old and 0.42 for those ≥75 years old) and nodal disease (HR 0.15 for N0 and 0.33 for N1 disease).11 In February 2018, apalutamide was the first agent to gain US Food and Drug Administration (FDA) approval for use in nmCRPC.19
Enzalutamide in non-metastatic castrate-resistant prostate cancer
Shortly after the start of the SPARTAN trial, the Safety and efficacy study of enzalutamide in patients with non-metastatic castration-resistant prostate cancer (PROSPER) trial also began recruiting a similar demographic of participants into its trial evaluating enzalutamide in nmCRPC, a drug that was already approved for the treatment of mCRPC.20 The PROSPER trial enrolled 1,401 patients with N0 nmCRPC and randomized them in a 2:1 ratio to a daily regimen of 160 mg of enzalutamide or placebo, respectively. At the time of writing, the third preplanned interim analysis showed an increased median MFS by 21.9 months in the enzaluatmide group when compared with the placebo (36.6 versus 14.7 months, respectively). Additionally, the analysis reports a 27% lower risk of death than with placebo.13 The PROSPER trial confirmed the side-effect profile of previous enzalutamide trials; the most common AEs related to enzalutamide were fatigue, musculoskeletal events, falls, fractures and hypertension.13 In July 2018, enzalutamide was approved for use in patients with nmCRPC by the FDA.21
Darolutamide in non-metastatic castrate-resistant prostate cancer
Darolutamide has the same AR inhibitory activities as apalutamide and enzalutamide, but additionally reduces the activity of AR variants.16 Daroluatamide was the most recent next-generation AR antagonist to be trialled in patients with nmCRPC in the Efficacy and safety study of darolutamide (ODM-201) in men with high-risk non-metastatic castration-resistant prostate cancer (ARAMIS) trial, which began recruiting patients in late 2014.12 The ARAMIS trial was similar to the SPARTAN and PROSPER trials in the demographic of patients allowed on study; however, one of the key differences of the ARAMIS trial was the inclusion of participants with a previous history of a seizure disorder or a concurrent condition that predisposed them to a seizure owing to lower blood–brain barrier penetrance.22 With these criteria, the ARAMIS study enrolled 1,509 patients 2:1 to receive active drug (twice-daily 600 mg darolutamide) or placebo. After meeting the primary endpoint of MFS, the trial was unblinded. Since then, the most recent preplanned interim analysis was published in September 2020, which showed a significantly reduced risk of death (31%) with darolutamide when compared with placebo, and a previously reported increase in median MFS of 22 months on darolutamide compared with placebo (40.4 versus 18.4 months, respectively). The AE profile of darolutamide is like that of other next-generation AR antagonists, and includes an increased risk of fatigue; however, darolutamide was not associated with a higher incidence of falls or fractures.12,18 Of note, it is not possible to do cross-trial comparisons, and head-to-head data comparing the agents are not yet available. Darolutamide was approved by the FDA for use in patients with nmCRPC in July 2019.23
Considerations in therapy selection
Although a direct comparator trial between the three next-generation AR antagonists does not exist, groups have analysed the SPARTAN, PROSPER and ARAMIS trials to this aim. When analysed holistically, the three drugs share significant efficacy by measure of MFS; all three increased MFS by approximately 2 years MFS compared with placebo.24,25 At a more granular level, a recent network meta-analysis showed that enzalutamide had a slight advantage in MFS and time to PSA progression over darolutamide, but no significant differences were observed between enzalutamide and apalutamide.26 This sort of analysis is insufficient to inform treatment selection.
In the context of potential AEs, the three have similar profiles; however, there are several minor differences that may be helpful for certain patient subgroups. The most common side effects include fatigue, hypertension, arthralgia, nausea and diarrhoea. Notably, by trial design and known mechanism of action data, darolutamide is preferred for patients with seizure disorders or comorbidities that increase the risk of seizures. The AE profile suggests that darolutamide is also not associated with an increased risk of falls and fractures.11,13,18 An upcoming clinical trial will help describe the tolerability of darolutamide relative to enzalutamide (NCT04157088).
Lastly, as innovations in early cancer detection and intervention improve, the population of patients with nmCRPC and other later-stage cancers is getting older. With this, the risk of drug–drug interactions owing to polypharmacy increases.27 Enzalutamide is known to induce many cytochrome enzymes, including CYP3A4, which has a strong potential to cause drug–drug interactions. The skilled expertise of a pharmacist is helpful in navigating this issue when selecting next-generation AR antagonists as the preferred therapy of choice.28
Imaging considerations
In all three next-generation trials, conventional CT or magnetic resonance (MR) imaging was used to evaluate soft-tissue disease and whole-body radionucleotide scintigraphy to evaluate bone disease.11,18,20 More sensitive imaging modalities are now available for the earlier detection of metastases, such as prostate-specific membrane antigen (PSMA) positron emission tomography (PET)-CT, which may affect classification of patients with metastatic disease to include oligometastatic disease. Increased use of PSMA PET-CT or other sensitive imaging techniques can contribute to the idea of stage migration in which a patient is risk-stratified to a higher disease stage (e.g. from locally advanced to oligometastatic). This shift in stage changes the treatment options for these patients and causes a paradoxical increase in survival of both patient groups. This phenomenon is colloquially known as the Will Rogers phenomenon.29,30 With this in mind, further investigations comparing the use of conventional and higher-sensitivity imaging to stratify staging and influence treatment decisions can help guide our understating of the role of new imaging modalities in prostate cancer.
Future directions
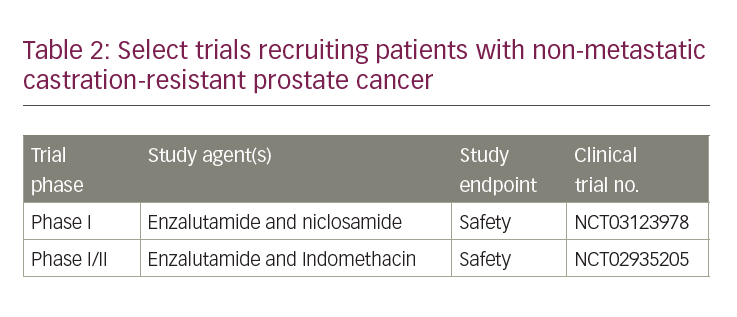
With the emergence of more effective therapies for castrate-resistant prostate cancer, and improvements in imaging, treatment of nmCRPC continues to evolve. Currently available treatments for mCRPC have now entered the nmCRPC space, specifically next-generation AR antagonists as described. Other drugs for mCRPC, such as CYP17 inhibitors and poly ADP ribose polymerase inhibitors, are entering the space of non-metastatic prostate cancer. Next-generation AR antagonists are working their way into the castration-sensitive non-metastatic setting and may be ineffective by the time a patient develops nmCRPC. With continued advances in imaging, the nmCRPC disease state may cease to exist. Recruiting studies in nmCRPC are investigating drugs that have the potential to enhance treatment response to enzalutamide (Table 2).
Conclusions
In recent years, the standard of care for nmCRPC has changed, resulting in prolonged time to metastatic disease, as well as improved overall survival. Cumulative exposure to next-generation AR antagonists has increased from a median of 8.3 months in mCRPC after chemotherapy to a median of 16.6 months in mCRPC in chemotherapy-naïve patients and now 18.4 months in nmCRPC.20,31 Investigations into how this increased survival will impact cancer biology and general health in these patients is underway.














