Prostate cancer (PC) has probably afflicted males in mid- to late life since humans evolved; evidence indicates that it has been a likely cause of death for thousands of years. DNA analysis of bone marrow from preserved human remains of a Scythian king, aged 40–50 years, found at an archaeological site in Siberia and dating from around 700BC indicates that he died from metastatic prostate carcinoma (mPC).1 Since that time, up until around the 1940s, treatments for the disease remained largely unchanged, concentrating on ameliorating the symptoms but having little or no effect on outcomes.2 PC is the most commonly diagnosed cancer in men, its incidence ranging from 34–100 cases/100,000 in Europe and 125/100,000 in the US.3 Incidence of the disease has risen over the past 100 years due in part to its recognition as a distinct disease in the early 20th century and as a likely result of Western lifestyles.2
In the 1940s, mPC was first shown to respond to surgical androgen ablation therapy and subsequently, oestrogen castration therapy emerged as the first effective medical treatment. Androgen deprivation therapy (ADT) continues to be a key therapy for PC; even patients with widespread metastatic disease respond well to it.2,4 Results from the Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) trial, the largest PC trial to date (n=2,962), showed that patients respond to ADT for an average of 12 months, after which, biochemical progression is usually the first indication of loss of disease control.5 Metastatic castration-resistant prostate cancer (mCRPC) is an incurable condition and, in spite of various developments in treatment, the median survival is currently only 22 months.6 There is therefore a relatively short window of opportunity during which PC can be treated to maximise therapy and deliver all the life-prolonging treatments available to patients.
Bone-seeking radionuclides in the treatment of prostate cancer
Among various isotopes, radioactive phosphorus (32P) has been used as a treatment that would accumulate in the bones of patients with mPC and kill or inhibit potentially tumour-generating cells.7 32P proved not to be a successful isotope for cancer treatment; however, several others have proven more suitable. These fall into two groups: calcium analogues (strontium-89 [89Sr] [beta-emitter]) and phosphate analogues (samarium-153, rhenium-186, rhenium-188 and 32P [all beta-emitters]). These treatments exploit the abnormal bone metabolism seen in various cancers, in which the radionuclides behave like calcium or phosphate, are attracted to the bone from the bloodstream and deliver a radioactive beta particle ‘payload’.8–10 The clinical trial evidence supporting these radionuclides in patients with PC is summarised in Table 1.11–20 The studies listed in Table 1 are mostly small or the proportion of patients with PC, among other cancer types, is low. The major endpoint for these beta-emitters in each study was pain response rather than efficacy against cancer. Where metastatic cancer results in bone dysfunction, pain can be a serious consequence. Diminishing this effect is highly important and is a major endpoint for quality of life in clinical trials.9,10,21,22
For beta-emitters in cancer treatment pain response rates may be as high as 70% but are mostly 30–50%; dose-limiting toxicity to the bone marrow and platelets is an important restriction on increasing drug exposure to improve efficacy.21–23 Beta-emitters do not confer any survival benefit; these treatments are mostly palliative and help diminish bone pain. There are also radioprotection issues in using beta-emitters; excreted radionuclides can be hazardous, particularly in patients who are incontinent.
Dawn of targeted alpha therapy
Following the discovery of radium by Marie and Pierre Curie in 1898, the element was soon applied to the treatment of cancer in 1906 in the form of inserted metallic rods (brachytherapy). Alpha-emitters have been considered as treatments for cancer since the early 20th century but it was not until relatively recently that advances in targeted delivery of radionuclides, radionuclide conjugation chemistry and increased availability allowed the development of suitable treatments and the initiation of clinical trials. Over the past 20 years early trials have been conducted with bismuth-213 (213Bi), astatine-211 (211At), actinium-225 (225Ac) and thorium-227 (227Th).24–26 In addition, monoclonal antibody-mediated targeted alpha therapies (TATs) including 225Ac-labelled anti-CD33, 213Bi-labelled anti-epidermal growth factor receptor (EGFR) and 211At-labelled anti-CD45 are also in development. Furthermore, peptide- or small molecule-mediated TATs such as 225Ac-PSMA-617, lead-203(203Pb)-AR-RMX and radium-223 (223Ra)-labelled barium ferrite nanoparticles are in early development27,28 and have interesting potential in improving the prognosis in mPC and some other cancers. As yet, the only TAT that has been approved for clinical use in mPC is 223Ra (Xofigo®, Bayer, Leverkusen, Germany).29–33
Radium-223
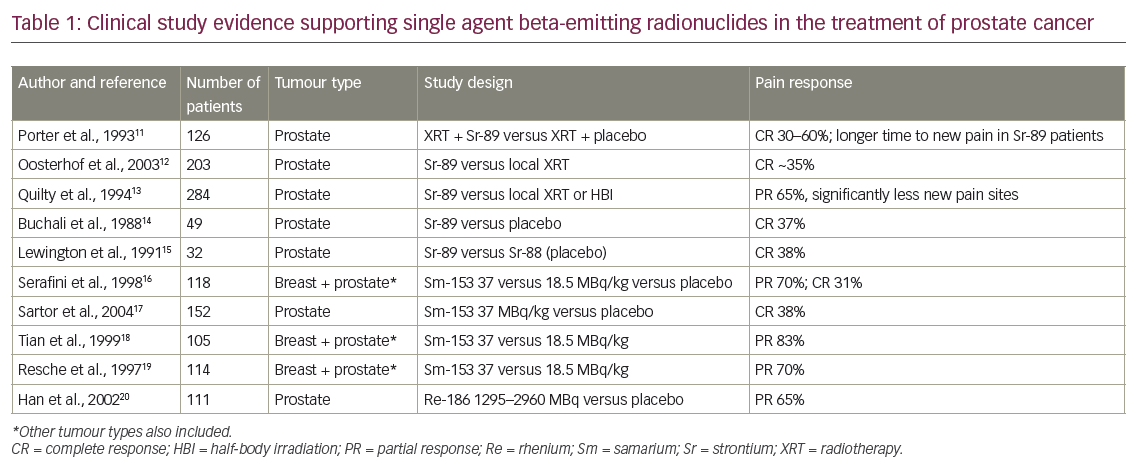
223Ra is an alpha-emitter and calcium mimetic that has been developed as a molecular-mediated TAT. 223Ra has a half-life of 11.43 days, which is optimal for clinical use and allows for distribution from a central production site to treatment centres worldwide. This radionuclide decays via a series of seven progenies to a stable isotope of lead (207Pb) which is considered to be biologically safe. During this decay, 93.5% of the energy is emitted as alpha particles, <4% as beta particles and <2% as gamma or X-rays.34 This radiation profile enables simple imaging and measurement on standard dose calibrators. Gamma imaging after 223Ra indicates a powerful dose of radiation directly to the sources of bone metastases (Figure 1). 223Ra is cleared via the gastrointestinal (GI) tract, unlike the beta-emitters. Gamma radiation scans demonstrate concentration of radiation within the large intestine 2 days after dosing, but by 6 days it has mostly been eliminated.30,32,33
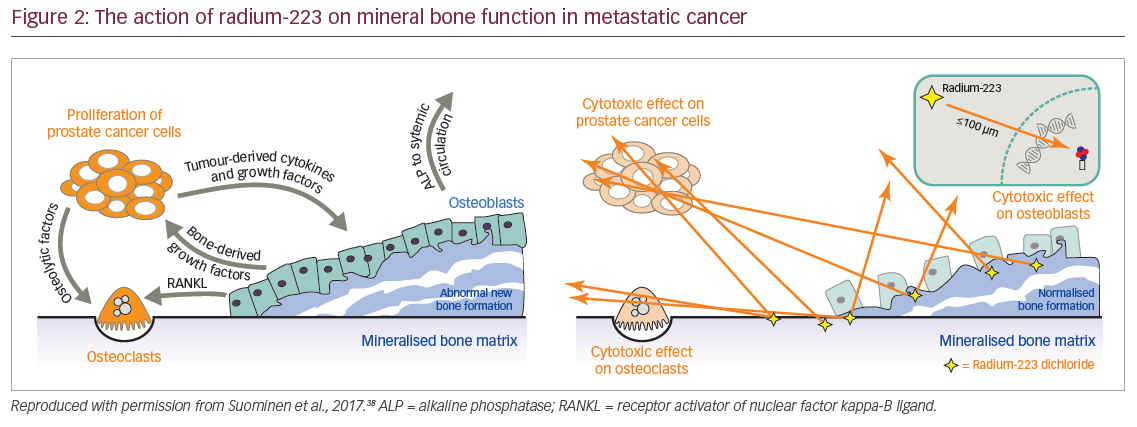
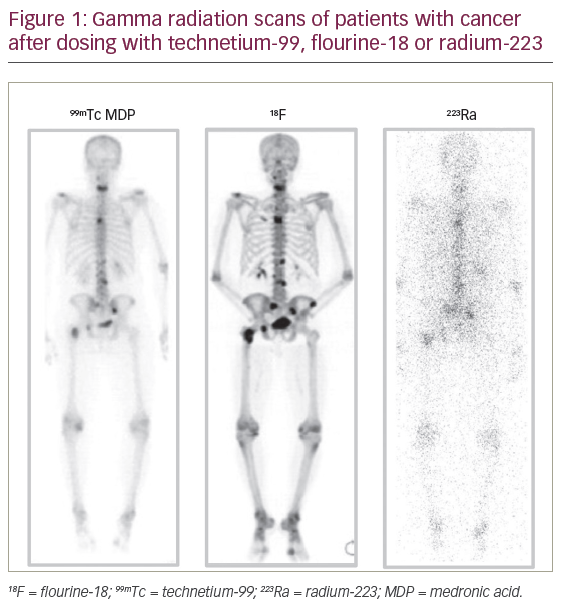
Alpha-emitters such as 223Ra deliver higher energy radiation doses to a smaller target area than beta-emitters. Consequently, this radiation requires fewer hits to DNA molecules to kill cells. DNA damage with alpha-emitters tends to be double-strand breaks which are difficult to repair compared with single-strand breaks that tend to be made by beta-emitters which are repairable (Table 2).26,35–37 When 223Ra reaches mineral bone it kills target mPC cells but also affects the entire bone microenvironment, particularly the osteoclasts and osteoblasts which, in metastatic cancer, are involved in abnormal bone formation. In addition, this treatment inhibits growth factors that drive the cancer in a vicious feedback loop. 223Ra therefore has a dual action in both targeting mPC cells and helping stabilise the bone microenvironment by inhibiting bone dysfunction and consequently reducing pain (Figure 2).38 For these reasons 223Ra has life-extending efficacy that the previous generation of beta-emitting radionuclides did not demonstrate.
Radium-223 clinical trials
The clinical development program of 223Ra has involved a series of phase I, II and III studies.29 The phase II, dose-ranging BC1-03 trial (n=100) showed the tolerability of single doses of differing amounts of 223Ra (5, 25, 50, or 100 kBq/kg) in castration-resistant prostate cancer (CRPC).39 The phase II BC1-02 trial (n=64) showed the tolerability of multiple doses of 223Ra (4 × 50 kBq/kg) and showed significantly decreased prostate specific antigen progression compared with placebo (p=0.048).40 The phase II BC1-04 study (n=122) also evaluated multiple doses of 223Ra (3 × 25, 50, or 80 kBq/kg) and showed that normalisation of alkaline phosphatase (ALP) was associated with a significantly better survival compared with those who did not show such normalisation.41
The pivotal ALpharadin in SYMPtomatic Prostate Cancer (ALSYMPCA) trial (n=921) is the largest treatment study of PC to date. It is an international, randomised, double blind, phase III clinical study that aimed to evaluate the efficacy and safety of 223Ra dichloride in patients with hormone-refractory PC and skeletal metastases.31 The inclusion criteria specified a confirmed diagnosis of CRPC, at least two bone metastases, no visceral metastases and previous treatment with or unsuitable for docetaxel. Patients were randomised 2:1 to receive six injections of 223Ra(50 kBq/kg intravenously) or matching placebo (one injection every weeks). All patients also received the best standard of care, (defined as the routine care provided at each centre [e.g., local external-beam radiation therapy or treatment with glucocorticoids, antiandrogens, ketoconazole, or oestrogens such as diethylstilbestrol or estramustine]). This care could not include cytotoxic chemotherapy or another radionuclide. After the initial treatment phase there was a 24-month follow-up phase during which patients received no further study treatment but were assessed at 4-month intervals.
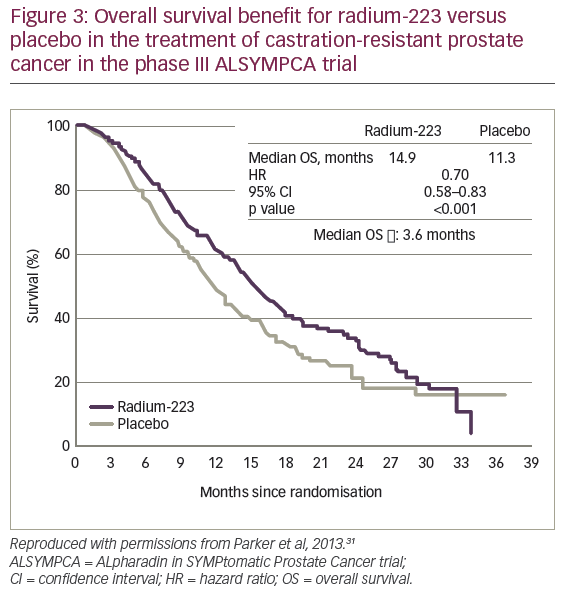
For the primary endpoint in the ALSYMPCA trial, median overall survival (OS) was 14.9 months for 223Ra versus 11.3 months for placebo (hazard ratio [HR] 0.70, 95% confidence interval [CI]: 0.58–0.83, p<0.001) with a median treatment difference of 3.6 months (Figure 3).31 This showed a marked advantage with 223Ra treatment and justified clinical use of the treatment in CRPC. Among secondary endpoints, there was a marked delay in median time to first skeletal event (15.6 months for 223Ra versus 9.8 months for placebo; HR: 0.66; 95% CI: 0.52–0.83, p<0.001). Among adverse events, thrombocytopaenia was more common with 223Ra than placebo (all grades: 12% versus 6%, grades 3 or 4: 6% versus 2%). Fornon-haematological events there was more diarrhoea of all grades with 223Ra (25% versus 15%) but there was no difference in grade 3 or 4 diarrhoea (2% versus 2%) and this was easily treated. Other expected adverse events – particularly nausea, vomiting and constipation – occurred at similar frequencies with 223Ra and placebo.31
Another study investigated 223Ra treatment of CRPC in an international open access program and included patients who were asymptomatic at baseline. This was a phase IIIb open-label study that enrolled patients with CRPC (n=696) who were treated with up to six injections of 223Ra (50 kBq/kg) at 4-weekly intervals.42 The results showed OS was longer in patients with baseline ALP<the upper limit of normal (ULN) than in those with baseline ALP≥ULN (16 months versus 12 months). Median OS was also longer in patients who received 223Ra plus denosumab (15 months) than in patients who received 223Ra without denosumab (13 months). The most frequent grade 3 or worse treatment-emergent adverse events were anaemia (5%), thrombocytopaenia (2%), neutropaenia (1%) and leucopenia (1%). Serious adverse events of any grade were reported in 35% of patients.
It should be noted that preliminary data from the ERA-223 trial (NCT02043678), a randomised, double-blind, placebo-controlled study, showed that there was an increased incidence of fractures (24% versus 7%) and deaths (27% versus 20%) among patients receiving 223Ra when administered concurrently in combination with abiraterone acetate and prednisone/prednisolone (n=4,010), compared with patients receiving placebo in combination with abiraterone acetate and prednisone/prednisolone (n=405). This study in asymptomatic or mildly symptomatic chemotherapy-naive patients with bone predominant mCRPC was unblinded early based on an independent data monitoring committee recommendation. The manufacturer and European regulatory authorities advise that whilst further investigation of the implications of these findings is in progress, patients should not be treated with 223Ra dichloride in combination with abiraterone acetate and prednisone/prednisolone with mCRPC.43–45
New treatment paradigms in castration-resistant prostate cancer
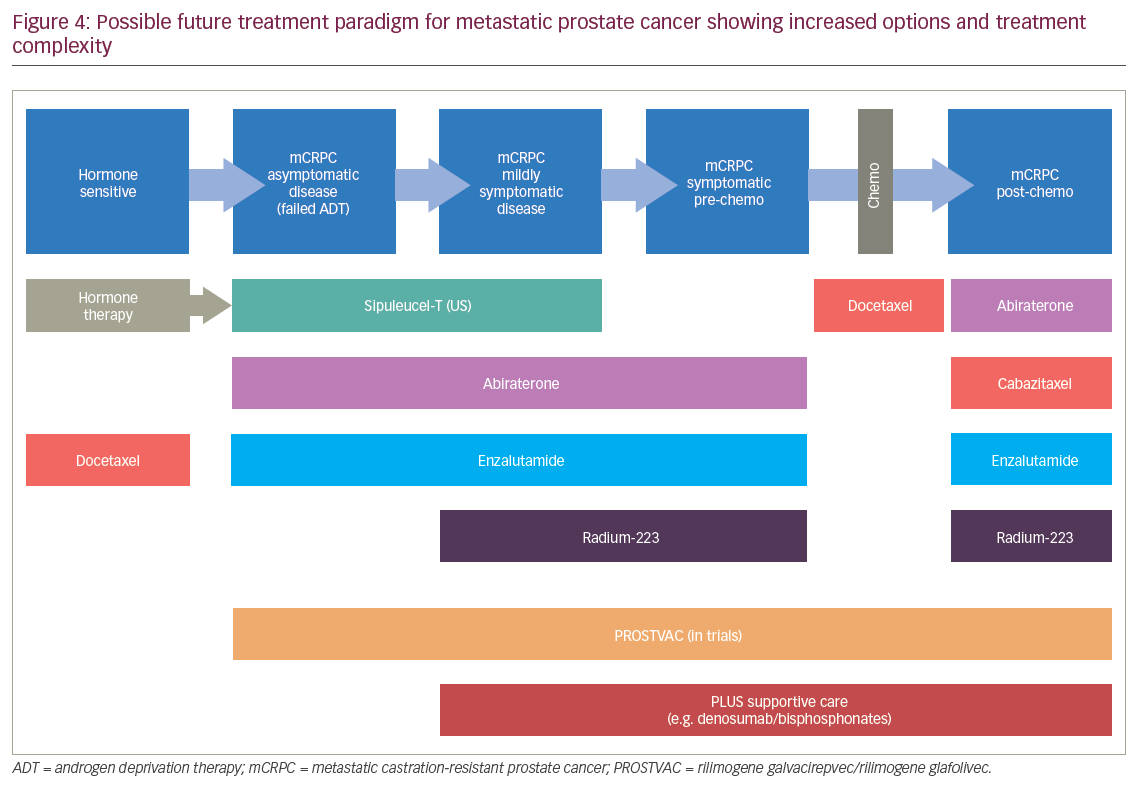
In recent years, a series of clinical trials have changed the treatment paradigm of CRPC. These have all demonstrated survival benefit of drugs and combinations including: abiraterone, enzalutamide, prednisone with cabazitaxel or mitoxantrone, docetaxel with prednisone or mitoxantrone, 223Ra, sipuleucel-T immunotherapy (US only) and docetaxel/zoledronic acid with hormone therapy.5,31,46–53 As a result of these positive findings, the treatment paradigm for mPC is increasingly complex as multiple different therapeutic options and combinations are becoming available (Figure 4). These advances have extended survival but they also pose challenges in terms of endpoint selection in clinical trials and deciding what control arm to use. These advances also increase the likely costs of treatment. The cost and suffering in mPC can potentially be reduced by moving the treatment ‘upstream’ i.e., starting treatment earlier. The advantage of such a move was demonstrated in the STAMPEDE trial, in which patients who presented with mPC at the time of diagnosis – before the disease became castration resistant – were given six cycles of docetaxel with castration therapy. This extended survival by almost 2 years, which is an astonishing improvement of survival in patients with locally advanced or metastatic disease.5
Similar improvements in survival may also be possible by treating early with TAT. Such an approach is currently under investigation in the Androgen Deprivation Therapy, Pelvic Radiotherapy and Radium-223 for presentation T1-4 N/1 M1B (ADRRAD) trial.54 In this phase I study, patients presenting with de novo mPC with bone metastases begin treatment with ADT (3 months neo-adjuvant luteinising hormone-releasing hormone analog [LHRHa]), which continues during subsequent treatment. They receive six cycles of docetaxel and are then given radiation treatment (74Gy in 37 fractions by volumetric modulated arc radiotherapy [VMAT] to prostate and pelvis) and six cycles of 223Ra (55 kBq/kg) commencing at the time of radiotherapy. This ‘upstream’ treatment may have a greater ‘downstream’ effect than delayed therapy but may also take advantage of a stage when the cancer is well controlled and prostate-specific antigen (PSA) levels are low during internal and external radiation but there is still good uptake of 223Ra into the bones. This trial is ongoing and the results are awaited with interest.
An alternative experimental approach to advanced PC treatment with radionuclides is the use of 225Ac-labelled prostate-specific membrane antigen ligand (PSMA).55 In a small study, experience with two initial patients showed that this treatment reduced PSA to undetectable levels and positron emission tomography (PET) imaging using 68Ga-PSMA-11 indicated a complete response. This is a next generation TAT in that it broadens the molecular-mediated use of radium by linking it to a tumour-specific agent and the authors believe it has strong potential to significantly benefit patients with advanced-stage PC.
Another alpha-emitter with potential in the treatment of PC is 227Th. This isotope decays via six intermediate progenies to 207Pb with a half-life of19 days and has a chemistry that is suitable for conjugation with antibodies. Targeted thorium conjugates (TTC) are under preclinical investigation in the treatment of various tumours including non-Hodgkin’s lymphoma, breast, colorectal, gastric, lung and prostate cancers.25 In vitro and animal model studies have shown CD22-TTC treatment significantly inhibited tumour growth and improved survival in lymphoma models.56 PSMA-TTC has shown selective and significant anti-tumour efficacy in PC xenograft models,57 and HER2-TTC has demonstrated significant anti-tumour efficacy in breast and lung cancer models.58 In addition, 227Th-conjugate, FGFR2-TTC has inhibited tumour growth in colorectal and in gastric cancer models59 and mesothelin-TTC has shown in vivo anti-tumour potency in a bone orthotopic metastatic xenograft model.60
Discussion and conclusions
Advances in treatments for mPC, mostly over the past 4 years, have resulted in significantly delayed progression of disease and extended survival. There is currently a huge clinical trial effort into various treatments for mPC and whilst the disease is not yet curable, these initiatives are improving prognoses and quality of life. Among newly emerging treatments, the TAT class, the first of which is 223Ra, has demonstrated favourable tolerability and improved efficacy in terms of treating abnormal bone function and extending survival. TATs are attractive to oncologists treating patients with mPC in that they show advantages over the previously used beta-emitters. These include delivery of higher energy radiation doses to tissues and killing tumour cells more effectively. As such, these radionuclides are likely to be used increasingly as their value in mPC treatment becomes more widely appreciated and they are accepted by more oncologists and pharmacies.
The clinical trials of 223Ra for the treatment mPC produced some impressive evidence. The most notable of this comes from the ALSYMPCA trial, which included the largest patient population of any trial in PC and showed significantly extended overall survival for 223Ra versus placebo (median 14.9 versus 11.3 months, respectively, p<0.001).31 This study also showed a significant delay in times to first skeletal event (median 15.6 versus 9.8 months, respectively, p<0.001). The safety profile was favourable with little increase in grade 3 or 4 haematological events and only a small increase in GI events with 223Ra. On the strength of these data, 223Ra was approved for use in Europe, the US, Japan and 40 other countries.61,62
Published real-life clinical experience with 223Ra in mPC is now emerging and it generally supports the clinical trial data. At one institution, a retrospective audit of 58 patient records showed that the treatment is effective in the clinic, and showed delayed progression-free survival, improved overall survival and decreased analgesic requirements whilst showing good tolerability and toxicity profiles, even though it was mainly being used as a third-line therapy or higher.63 In this analysis and in clinical trials, long-term toxicity of 223Ra after 1.5 years or longer was confined to haematological events, which were mainly mild and with no myelosuppression. The Observational Study for the Evaluation of Long-term Safety of Radium-223 Used for the Treatment of Metastatic Castration Resistant Prostate Cancer (REASSURE) (NCT02141438,n= ~1,440) is now in progress and aims to monitor long-term toxicities of 223Ra in the treatment of mPC and will also monitor whether there are any secondary malignancies that have not been seen to date.
Several study authors have commented that the optimal position of 223Ra in the mPC treatment sequence needs to be more clearly defined. Some oncologists believe that the efficacy of the treatment would be considerably greater if it was commenced at an earlier stage before castration resistance has developed. There is some reluctance to use radionuclides in mPC since earlier attempts using strontium failed to improve survival.64,65 However, TAT has distinguished itself as an overall survival class of therapies. In addition, the choice and sequencing of therapy in mPC is becoming more challenging as the number of treatments increases and the paradigm becomes more complex.
Knowledge of the pathophysiology of mPC is rapidly increasing and this is informing more successful treatment strategies. In future, oncologists will have an expanding choice of treatments for this cancer and will increasingly need to tailor therapies to the individual patient and their specific disease stage rather than applying the same schedule to all. Oncologists may also need to be more proactive in moving ‘late-stage’ treatments such as 223Ra to an earlier stage to improve outcomes, delay progression and maximise survival times.














