Osteosarcoma is the most common primary malignant neoplasm of bone in children and adolescents. It is characterised by the proliferation of malignant mesenchymal cells that are capable of producing osteoid or immature bone.1 About 800 new cases of osteosarcoma are diagnosed per year in the US. Half of these cases occur in people under the age of 20, making it the sixth most common malignancy in adolescents and young adults.2 Prior to 1970, the prognosis for patients with osteosarcoma was dismal, with a 10–20 % overall survival (OS) rate for patients with localised disease, despite being treated with aggressive surgery. The majority of these patients developed and eventually died from overt metastatic disease. Survival for patients with osteosarcoma has improved dramatically over the past three decades with the addition of systemic chemotherapy to surgical resection. Today, 65–75 % of patients with localised disease will become long-term survivors.3 In this article, we will review the current chemotherapeutic treatments of osteosarcoma, controversies regarding chemotherapy use and emerging promising developments in the management of this aggressive neoplasm.
Chemotherapy in Osteosarcoma Overview
Before the introduction of adjuvant systemic chemotherapy, patients with osteosarcoma had less than a 20 % OS. Most patients developed locally recurrent or metastatic disease, presumably from microscopic subclinical metastatic disease that was present at the time of diagnosis.4 With modern multimodality therapy combining systemic chemotherapy and complete surgery, the cure rate now approaches over 70 % for patients with non-metastatic osteosarcoma.5 Numerous trials have been performed in the past 30 years that have investigated the utility of adjuvant chemotherapy in patients with osteosarcoma. These trials have identified high-dose methotrexate (MTX), cisplatin, doxorubicin, ifosfamide and etoposide as active cytotoxic agents.6–10 Combinations of these agents – although mostly empirical – now make up the cornerstone of treatment. Some of the notable trials using combination chemotherapy over the past 10 years are summarised in Table 1. Although the exact combination, dose and schedule of chemotherapeutic agents is still debated, several randomised controlled trials have clearly demonstrated a significant survival benefit of systemic therapy in the management of osteosarcoma.11,12
Chemotherapy in Osteosarcoma – Concepts and Controversies Rationale for Neoadjuvant Chemotherapy
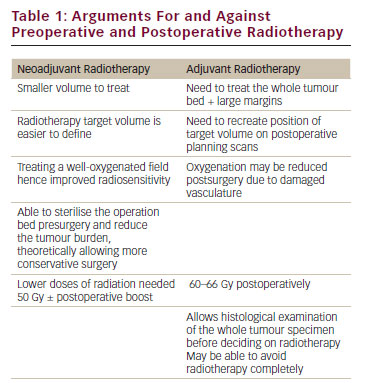
The rationale for administering neoadjuvant chemotherapy was initially based on the development of limb salvage procedures and custom-made endoprostheses that took several months to manufacture. Chemotherapy was employed to bridge the gap from biopsy to resection.13,14 However, it was soon discovered that neoadjuvant therapy might not improve only limb salvage rates, but also survival. This hypothesis was confirmed in a randomised study (POG-8651) conducted by the Pediatric Oncology Group (POG) from 1986 to 1993. POG-8651 compared surgery followed by adjuvant chemotherapy versus neo-adjuvant chemotherapy followed by surgery. The event-free survival (EFS) was similar in both groups – 65 % for immediate surgery and 61 % for neoadjuvant therapy – and the limb salvage rates were similar in both groups (50–55 %), implying that there was no significant improvement in outcome with neoadjuvant versus adjuvant chemotherapy in localised osteosarcoma.15 Another rationale for using neoadjuvant chemotherapy is the capability of individualising therapy based on tumour response. It has been reported from numerous trials that histological response with tumour necrosis greater than 90 % confers a better prognosis.15–18Intensifying Chemotherapy Based on Histological Response
The strategy of intensifying or altering post-operative therapy based on poor tumour necrosis was used successfully in the 1980s by investigators at the Memorial Sloan Kettering Cancer Center (T10 trial) and later confirmed by the Rizzoli Institute.19,20 However, the results of these trials were not replicated in other large co-operative group studies.17,21,22 The question of intensification and individualisation of therapy based on tumour necrosis is currently being investigated in a large co-operative trial through the European and American Osteosarcoma Study Group (EURAMOS1, AOST0331, ClinicalTrials.gov/NCT00134030). This is a multinational collaboration of the Children’s Oncology Group (COG), Cooperative Osteosarcoma Study Group (COSS), the Scandinavian Sarcoma Group (SSG) and the European Osteosarcoma Intergroup (EOI). In this study, all patients receive two cycles of high-dose MTX x2, cisplatin and doxorubicin prior to surgery. Patients with poor necrosis as defined by less than 90 % of the resected sample are randomised to receive high-dose MTX, cisplatin and doxorubicin with or without the addition of ifosfamide and etoposide. On the other hand, patients with a good response (>90 % necrosis) are continued on high-dose MTX, cisplatin and doxorubicin then randomised to a maintenance arm with pegylated interferon alpha (IFN-α). This trial has recently closed to accrual with over 2,000 localised osteosarcoma patients as of March 2011.
High-dose Methotrexate
The folate antagonists were among the first chemotherapeutic agents to be developed, starting with aminopterin in 1948. Two decades later, in 1972, Norman Jaffe reported the successful administration of high-dose MTX (HDMTX) in combination with leucovorin (citrovorum) rescue in 10 patients with metastatic osteosarcoma. A complete regression was obtained in two patients and a partial regression in two others.23 This seminal paper, although initially subject to much criticism, outlined the cornerstone of therapy for osteosarcoma for decades to come. Soon afterwards, Gerald Rosen and colleagues combined HDMTX with doxorubicin to create the standard adjuvant regimen currently used in childhood osteosarcoma.24
Since then, the use of HDMTX has been intermittently subject to intense scrutiny. In general, the term HDMTX corresponds to a dose greater than 500 mg/m2. However, in osteosarcoma doses of 8–12 g/m2 are used as standard, necessitating the use of leucovorin rescue to bypass the metabolic block induced by MTX. Interestingly, the rationale behind leucovorin rescuing normal over cancer cells is not totally understood. It is widely recognised that a peak serum MTX level of 750 μmol/mol or greater is a prerequisite to successful therapy and that inability to achieve this is associated with a poorer prognosis.25 On the other hand, it has been reported in a smaller series that patients in whom a greater than 1,500 μmol/mol mean peak serum MTX concentration was achieved had a worse outcome.26 Despite the absence of a randomised trial evaluating osteosarcoma treatment with and without HDMTX, it is generally acknowledged that it is ‘time for final acceptance’ of MTX in our standard osteosarcoma armamentarium.27
The Addition of Ifosfamide
Ifosfamide, the nitrogen mustard alkylating agent, is clearly an active agent either alone or in combination with etoposide in recurrent and/or metastatic osteosarcoma with a response rate of over 30 %.28 However, the addition of ifosfamide with or without etoposide to the three-drug regimen of HDMTX, cisplatin and doxorubicin in the treatment of primary localised osteosarcoma is controversial and ifosfamide exposure is not without side effects. Two European groups, the Co-operative German–Austrian–Swiss Osteosarcoma Study Group (COSS) and The Rizzoli Orthopaedic Institute (IOR), have obtained favourable results with ifosfamide-containing regimens.29,30 However, in a recent large randomised controlled collaborative trial (INT-0133), the addition of ifosfamide to standard therapy was investigated (± the addition of the immunomodulator muramyl-tripeptide-ethanolamine [MTP-PE]). The addition of ifosfamide did not affect OS or EFS, while the addition of MTP-PE did result in a statistically significant improvement in OS (78 versus 70 %).31 The standard use of MTP-PE alone or in combination with ifosfamide will likely be the subject of future confirmatory trials.
The dosing of ifosfamide has varied in these various clinical trials from 9 to 18 g/m2 over three to five days. Clearly, higher doses of ifosfamide are associated with increased renal and haematological toxicities, although it is unknown whether higher doses of ifosfamide correlate with improved response rates and improved survival. When treating metastatic and/or recurrent disease, especially if having received ifosfamide in a previous regimen, we generally subscribe to the ‘more is better’ approach, using 14 g/m2.
Current Chemotherapeutic Regimen for Localised Osteosarcoma
There is no uniform chemotherapeutic regimen for osteosarcoma. Generally we consider the three-drug regimen consisting of HDMTX 12 g/m2 with leucovorin rescue, doxorubicin 75 mg/m2 over 48 hours, and cisplatin 120 mg/m2 over two days as standard therapy (see Table 2). The therapy given is identical to the standard arm of the AOST0331/EURAMOS1 trial where a 10-week induction phase precedes definitive surgical resection followed by a 28-week post-operative adjuvant phase. The addition of adjuvant ifosfamide should be utilised in the context of enrolment onto the AOST0331 clinical trial or on a case-by-case basis when patients are unable to receive one of the standard drugs in the three-drug regimen due to co-morbid conditions.
Treatment of Osteosarcoma in Adults
Osteosarcoma is most common in adolescents, though a second incidence peak is noted in the seventh and eighth decades of life. Many of these elderly cases are preceded by a previous malignancy with radiation therapy and Paget disease.32 The survival of patients less than age 50 is similar to children, adolescents and young adults. However, the OS is significantly decreased in older adults as less than 20 % of localised osteosarcoma patients in their sixties survive five years from diagnosis.32 This is likely due to a combination of the biology of the tumour and diminished tolerance of chemotherapy from co-morbid conditions and end-organ damage. Generally, we advocate the use of the same regimen as used in our younger patients with very judicious surveillance of renal, hepatic and cardiac function. In adults, as compared with adolescents, comparable rates of grade 4 toxicities have been noted with standard regimens as recently reported by the Rizzoli institute using HDMTX, cisplatin, doxorubicin and high-dose ifosfamide in osteosarcoma patients less than 40 years of age. However, delayed MTX excretion was associated with adults greater than 20 years old. This is likely from decreased renal function with older age.33 HDMTX administration in patients greater than age 50 can be challenging and, if not tolerated, is often omitted, using doxorubicin and cisplatin as a standard combination. OS with omission of MTX seems to be similar.34Chemotherapy in Recurrent and/or Metastatic Osteosarcoma
In contrast to the 60–70 % long-term survival of patients who present with localised osteosarcoma, patients with clinically evident metastatic disease at diagnosis have a poor prognosis. About 20 % of patients will present with metastatic osteosarcoma and the OS is reported to range from 10 to 40 %.37,38 There is no standard approach for treatment of patients with metastatic disease at diagnosis despite multiple clinical trials. Combination chemotherapy with doxorubicin, cisplatin, HDMTX, ifosfamide and etoposide is currently used at our institution for treatment. A POG trial with high-dose ifosfamide and etoposide induction therapy followed by adjuvant HDMTX, doxorubicin and cisplatin chemotherapy with lower-dose ifosfamide and etoposide had a 59 % overall response rate with a two-year projected survival of 39 % for lung-only and 58 % for bone-only involvement.10 Although these results appear to be superior to historical controls, the long-term survival data have not yet been reported. In most studies, however, patients with bony metastases fared poorly versus those with pulmonary metastases, and survival appears to inversely correlate with the number of metastases.37,39 Notwithstanding that there is no standard for treatment of metastatic disease at diagnosis, we recommend aggressive multi-agent chemotherapy, primary local control and metastasectomy if possible.
Thirty to forty per cent of patients with localised osteosarcoma will develop a recurrence in spite of incredibly aggressive chemotherapy and surgery. In several large series, the five-year survival has been reported between 23 and 29 %40,41 and complete surgery was required to achieve cure. In both studies, survival also correlated with the number of metastases at the time of recurrence as well as the recurrence-free interval. The use of chemotherapy in the adjuvant setting for metastatic osteosarcoma continues to be studied. Although controversial, many centres including ours advocate the use of adjuvant chemotherapy after metastasectomy when there is a solitary lung recurrence occurring less than 24 months from initial diagnosis, and a period of close observation for greater than 24 months from initial diagnosis.28,42 Ifosfamide 9–14 g/m2 with or without etoposide 100 mg/m2 is the favoured salvage regimen. Because there is no standard other than complete surgical metastasectomy, the decision of adjuvant chemotherapy is made on an individual basis. Other therapeutic approaches to the management of metastatic and/or recurrent disease include radiation to sites of metastases, especially when unresectable and other new promising investigational agents currently used in clinical trials (see Table 3).
Emerging Therapies
Over the past several decades, new chemotherapeutic agents have been added to the armamentarium of anticancer drugs. However, few new cytotoxic chemotherapeutic agents have shown activity or clinical benefit in osteosarcoma. The focus has been to develop targeted therapies for osteosarcoma to increase efficacy while minimising the bystander effects of our aggressive therapies.
Immunotherapy in Osteosarcoma
Immune approaches to osteosarcoma therapy continue to be investigated. Immunotherapy has been used in osteosarcoma therapy for several decades notably with the administration of IFN-α.43 The effect of maintenance pegylated IFN-α is being studied in the EURAMOS1 trial in patients with a good response to neoadjuvant chemotherapy. Another approach used the immuno-stimulant muramyl tripeptide phosphatidyl-ethanolamine (MTP-PE), which is derived from Bacille Calmette-Guerin and is a potent macrophage activator. Recently, addition of liposomal MTP-PE in combination with adjuvant chemotherapy resulted in a statistically significant increase in OS (78 %) versus standard combination chemotherapy (70 %).31 Other immune strategies have focused on generating T-cell responses by vaccination with the anti-idiotypic antibody mimicking CD55, a complement regulatory protein expressed by many solid tumours, including osteosarcoma.44,45 The use of dendritic cell vaccines to enhance cytotoxic T-cell activation is being evaluated in xenograft models as well.
Molecular Therapies
Small molecule therapy with inhibition of the Src kinase pathway involved in osteoclast activity has been shown to have antiproliferative and pro-apoptotic activity in osteosarcoma cell lines and xenograft models.46,47 The orally available Src tyrosine kinase inhibitor AZD0530 is under investigation in a phase II clinical trial in osteosarcoma with pulmonary recurrence post metastasectomy, conducted by the Sarcoma Alliance Research through Collaboration (SARC) global co-operative network (SARC012, NCT00752206). Other recent trials using small molecule biological therapy have focused on targeting the insulin-like growth factor receptor (IGFR) with the monoclonal antibody R1507 (SARC011, NCT00615680, osteosarcoma cohort closed to accrual) expressed in osteosarcoma and other sarcomas, as well as targeting human epidermal growth factor receptor 2 (HER-2) with the monoclonal antibody trastuzumab overexpressed in 30–40 % of osteosarcoma tumours (COG-AOST0121, NCT00023998, study completed). A summary of selected current open trials for osteosarcoma is listed in Table 3.Bisphosphonates
Zoledronic acid (ZOL) is a nitrogen-containing bisphosphonate widely used in the prevention and treatment of osteoporosis and treatment of other disorders of bone metabolism. ZOL inhibits bone resorption and has also been commonly used to reduce skeletal complications from bone metastases in a variety of malignancies. Additionally, it is recognised that ZOL has anti-neoplastic activity, the mechanisms of which are coming to light. Inhibiting the farnesyl pyrophosphate synthase enzyme in the mevalonate pathway causes changes in the post-translation modification of downstream G proteins (Ras, Rap1, Rho and Rab) and a subsequent decrease in cellular proliferation, adhesion and invasion. ZOL also appears to be a potent inhibitor of angiogenesis.48 In osteosarcoma, preclinical data suggest a promising role for ZOL in therapy of both localised and metastatic lesions. In various mouse models, ZOL has been shown to inhibit osteoblastic and osteolytic components of osteosarcoma lesions49 as well as reducing production of vascular endothelial growth factor (VEGF) and preventing lung metastases.50
A phase III randomised trial is under way in France studying combination chemotherapy and ZOL in localised osteosarcoma (NCT00470223) as well as a pilot feasibility and dose discovery trial analysing ZOL with concurrent chemotherapy in the treatment of newly diagnosed osteosarcoma in the US through the COG (AOST06P1/NCT00742924). Other bone-specific agents such as the anti-receptor activator of NF-kappa B (RANK) ligand monoclonal antibody denosumab, which has produced over 80 % response rates in giant cell tumours of bone, may show promise as anti-osteosarcoma agents in the near future.
Conclusions
The prognosis of localised osteosarcoma has improved dramatically over the past 30 years, with multimodality treatment of aggressive surgery and combination chemotherapy. There is no one standard chemotherapeutic regimen for osteosarcoma, though regimens with neoadjuvant HDMTX, doxorubicin and cisplatin, followed by definitive resection and then adjuvant chemotherapy with these aforementioned agents, is favoured by most centres. The addition of ifosfamide and etoposide is controversial, though they are clearly active in osteosarcoma. As such, the decision to use these agents for localised disease should be made on an individual basis or within the scope of a large international collaborative trial such as the EURAMOS I trial examining the role of the intensification of therapy with addition of ifosfamide and etoposide for patients with a poor response. Despite these advances for localised disease and with the development of newer chemotherapeutic agents, improvements in the survival curves for metastatic, refractory and recurrent osteosarcoma have been stagnant since the inception of combination chemotherapy. There are promising therapies emerging in trials and on the horizon, but a continued emphasis must be our understanding of the biology of osteosarcoma, with the goal of providing patients with new, molecularly targeted therapies.