Prostate cancer is the most common malignancy in the adult male population with incidence increasing with age. Approximately 15–40% of patients with prostate cancer will relapse after radical treatment for early disease.1 Salvage treatment is often considered for those with local relapse and increasingly for those with oligometastatic distant disease. The role of androgen deprivation in this population remains poorly defined, but is commonly used in patients with rapidly rising prostate specific antigen (PSA) or those at risk of systemic failure and death from lethal prostate cancer. There is, however, a high degree of interpatient heterogeneity and a significant proportion of patients live a number of years without developing metastatic disease. Studies to date have not focused on determining the relevance of the molecular complexity of this heterogeneity and have not addressed whether earlier systemic treatment beyond luteinizing hormone-releasing hormone analogue (LHRHa) is superior to later treatment. These issues clearly have major clinical and health economic implications as there is much evidence that many patients die with, and not from, prostate cancer.
A significant number of men with prostate cancer aged ≥75 years will experience biochemical relapse; the priority in these patients may be to do no harm, particularly in frailer patients. Clinical trials frequently report largely on younger men, with few patients aged ≥75 years recruited. Care must therefore be taken when extrapolating data to older patients, who often have more comorbidity and in whom the risk of treatment-related toxicity is higher. This is particularly important since large retrospective studies suggest that the median time to radiological evidence of metastasis on conventional imaging following biochemical recurrence is between 8 and 10 years.2 It is likely that during this period patients will not have their quality of life impacted by cancer symptoms, and it therefore follows that there is a significant population in whom continued observation beyond biochemical relapse is an appropriate option to minimise the risk of doing harm.
A major reason for treating non-metastatic (M0) patients early is the possibility of eradicating disease and attaining cure; achieving this goal is, however, limited by the fact that undoubtedly computed tomography (CT) and bone scans miss a large number of metastases due to poor sensitivity. Prostate-specific membrane antigen (PSMA)-positron emission tomography (PET) and multi-parametric magnetic resonance imaging (MRI) have significantly higher sensitivity than conventional imaging, but at low PSA levels the likelihood of detecting metastatic disease remains limited.3 Moreover, PSMA expression is not exclusive to prostate cancer cells and is not infrequently absent, particularly in the hormone sensitive setting. If we consider that 1 mm3 may contain 107 cancer cells, it is clear that even modern imaging will miss significant disease burden and that a large number of patients deemed M0 actually have metastases (M1) and are not curable with localised therapies. Nonetheless, it is possible that a proportion of M0 patients with a rising PSA have only localised recurrence or truly oligometastatic disease, but this population is likely hugely overestimated. In this highly selected group of true M0 patients, salvage radical prostatectomy post radiotherapy may result in clinical benefit, although more evidence to support this strategy is needed. Similarly, metastasis-directed therapy for oligometastatic disease may prolong androgen deprivation therapy (ADT)-free survival, but it is not yet known whether this translates into improved long-term outcomes.4
For men with M0 disease by conventional imaging and a short PSA doubling time despite castration, the PROSPER and SPARTAN trials recently reported improved metastasis-free survival with enzalutamide and apalutamide, respectively.5,6 Overall survival data are awaited and these trials have not yet addressed whether earlier treatment is superior to later treatment. This is an important issue since these drugs can cause significant cardiovascular, neurocognitive and bone morbidity, particularly in older men.
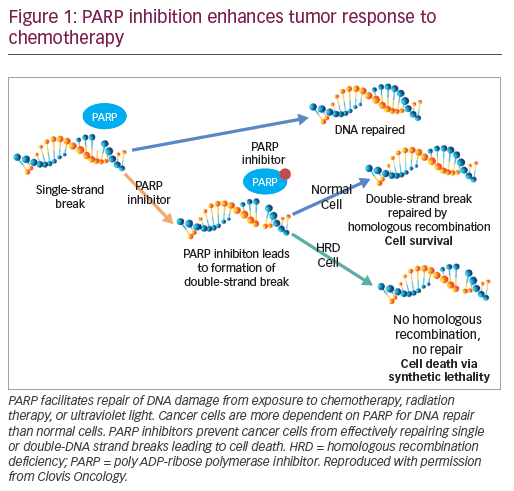
Key to advances in the field may be the development of more sensitive tools to identify prostate cancer cells, preferably assays that can detect single clonogenic tumour cells. More work is also needed to stratify prostate cancer subtypes based on those which are more, and less, likely to behave aggressively. Those with poorer prognostic features may warrant more aggressive upfront or adjuvant systemic therapy to decrease tumour burden, while those with better prognostic disease biology may benefit most by not being treated immediately despite rising PSA. This complexity has yet to be tackled in prospective trials of M0 patients, although recent progress in advanced disease studies offers promise. For example, in prostate cancers with DNA repair defects, studies indicate that BRCA1/2 germline mutant cancers may have worse outcomes when treated conventionally with surgery or radiation therapy.7 The increasing evidence that these cancers are highly sensitive to poly ADP ribose polymerase (PARP) inhibition and platinum-based chemotherapy provides an alternative treatment route to exploit.8 Similarly, phosphatase and tensin homolog (PTEN) loss is an early event in up to 50% of prostate cancers and is associated with worse overall survival and poor response to hormonal treatments alone; these patients may need more aggressive systemic therapies.9 Mismatch repair deficient and/or microsatellite unstable prostate cancers also appear to have a worse prognosis from the start of hormone therapy for advanced disease; these cancers may be better served by programmed cell death protein 1 (PD-1) and/or programmed death-ligand 1 (PD-L1) immune checkpoint inhibition.10,11
Overall, therefore, there remains insufficient level 1 evidence regarding how best to manage biochemical relapse, particularly in older men, patients with slow PSA doubling times and those with largely localised disease. The role and timing of adjuvant versus salvage radiotherapy post prostatectomy remains unclear; prospective data suggest that adjuvant radiotherapy in a high-risk group reduces the risk of biochemical relapse, but around 40% of patients will not recur at 10 years despite initial observation.12 Retrospective data suggest that early salvage radiotherapy (PSA<0.5) may improve metastasis-free survival, but prospective, randomised data are needed.13
In summary, a significant number of patients are destined never to die of prostate cancer despite biochemical failure after radical treatment. We need prospective data, and better biomarkers, to evaluate this population and the complexity of the hetereogeneity of these diseases. Next-generation imaging will improve the detection of early metastatic disease, and salvage treatments and systemic therapy may have a role to play in improving outcomes in selected subgroups. However, we must also focus on doing no harm and ensuring that this improved imaging does not lead to a large number of men unlikely to ever die from prostate cancer living with the knowledge and anxiety of the presence of metastatic disease.