The Premise of Targeted Drug Therapy
The Premise of Targeted Drug Therapy
Targeted molecular therapies comprise the newest frontier of oncology research.1–3 Recent investigations have concentrated on the development of novel treatments designed to interrupt cellular pathways crucial to oncogenesis and, in fact, these medications represent a large proportion of drugs undergoing evaluation in current and new clinical trials.2 Identification of the intracellular signals responsible for conferring malignant potential on tumor cells enables therapeutic discrimination between malignant and healthy cells.1,2 The expectation is that targeted therapies, in contrast to traditional chemotherapeutic agents, will provide enhanced patient outcomes and improved efficacy in the setting of superior, or at least complementary, toxicity profiles.3 Furthermore, targeted drugs provide alternative approaches to disruption of carcinogenesis, which may prove to be synergistic with conventional cancer therapies. While the advent of innovative treatment modalities for cancers has improved the landscape for affected patients, the overall response to therapy thus far has been modest.4
Monoclonal Antibodies
Monoclonal antibodies, first designed in 1975 by Kohler and Milstein,1 have evolved as a vital component of modern cancer therapy. Following selection of an appropriate target, monoclonal antibodies are developed within a murine host. Early versions of monoclonal antibodies were derived entirely from murine origins, providing a robust source of immunogenicity that necessarily limited their use in humans.1–3 These murine antibodies, identified by their suffix -momab, provoked human antimouse antibody (HAMA) responses against the murine proteins, thereby increasing rates of allergic reactions and decreasing therapeutic efficacy.1,3 Subsequent generations of monoclonal antibodies attempted to address the immunogenicity of these drugs by decreasing the proportion of murine proteins composing the antibody by linking human constant regions with variable murine antigenic sequences to simultaneously maintain both antibody activity and specificity. These human–murine chimeric antibodies (suffix -ximab) benefit from a lesser proportion of murine proteins than their fully mouse predecessors, with most antibodies composed of as little as 34% rodent material.2 These antibodies continue to elicit human immune reactions via the appearance of human antichimeric antibodies (HACAs), although the incidence is lower than with the earlier constructs.1,3 Reduction in the immunological response allows for superior patient tolerance, enhanced circulatory stability, and increased recruitment of immune mediators, leading to improved efficacy.1,2
Humanized antibodies (suffix -zumab) retain the complementary determining regions of the murine precursor, also known as the hypervariable region, with an entirely human underpinning. The final composition is approximately 95% human.2 Yet another subset of antibody response has been observed: human antihuman antibodies (HAHAs). Again, the frequency of such immune responses is much less than in previous antibody arrangements. Finally, a strategy for the virtual elimination of human hypersensitivity reactions following exposure to engineered monoclonal antibodies involves developing transgenic mice, and these fully human antibodies carry the suffix -mumab.1,2 The armamentarium of cancer therapy includes agents from each of these subtypes, which are either under investigation for use in humans or have already been awarded US Food and Drug Administration (FDA) approval for the treatment of certain malignancies.
Epidermal Growth Factor Receptor
Non-malignant cells develop in a regulated fashion using a series of intracellular signaling pathways that dictate typical cellular responses such as angiogenesis, proliferation, and cell survival. Preferential activation of these pathways leads to promotion of tumor growth, invasion, and metastasis. Receptor tyrosine kinase (RTK) activation mediates multiple intracellular signaling pathways, and dysregulation of the activation and expression of the components of this pathway is considered to be one etiology of tumorigenesis.5 The epidermal growth factor receptor (EGFR) is the gatekeeper of one such critical tyrosine kinase pathway and exemplifies a target for novel chemotherapeutics.
EGFR, a member of the RTK family erbB or HER, is a 170kDa transmembrane protein that serves to regulate a variety of downstream signals influencing tumorigenesis.2,3 The receptor is composed of an extracellular ligand-binding region, a transmembrane region, and an intracellular tyrosine kinsase domain that mediates intracellular signaling through the activation of cytoplasmic messengers. Following binding of the natural ligands (i.e. transforming growth factor-α [TGF-α] and EGF), the extracellular portion undergoes conformational change to facilitate homo- or heterodimerization with nearby EGFR or EGFR-related receptors. The dimerization promotes activation of the tyrosine kinase domain and initiation of the intracellular signaling cascade, as well as prompting internalization of the receptor for eventual degradation or recycling.3 Intracellular messengers recruit transcription factors in the nucleus and activate specified genes.
Downstream pathways activated by the ligand-binding cascade include those influencing apoptosis, proliferation, angiogenesis, metastasis, invasion, and survival. The EGFR is constitutively expressed in many normal epithelia, including in skin and hair follicles. In the skin EGFR plays a critical role in the maintenance of epithelial integrity.6 In contrast to non-malignant tissue, several epithelial cancers, including tumors of the colon, head and neck, breast, lung, kidney, bladder, brain, and pancreas, demonstrate overexpression of EGFR at varying rates.1–3 Overexpression of EGFR within malignant tissues has been associated with more aggressive disease and a poorer prognosis, as well as resistance to cytotoxic agents and impaired treatment responsiveness.1–3,7–11
Cetuximab
Cetuximab/C225 is a recombinant human–murine chimeric monoclonal immunoglobulin G (IgG)1 antibody that binds the EGFR with a 2-log higher affinity than native ligands such as TGF-α and EGF.1–3 Following binding of the antibody to the extracellular domain, the complex is internalized for eventual degradation. Unlike the effects of ligand binding, the binding of cetuximab does not stimulate tyrosine kinase activity and the dependent intracellular pathways remain quiescent. This process also results in impaired recycling of receptors to the cell surface, leading to overall receptor downregulation.1,2,12 Recent studies have demonstrated a tyrosine-kinase-independent, antibody-induced localization of the EGFR to the nucleus following endocytosis. A small fraction of receptors are transported to the nucleus via the endoplasmic reticulum, though what influence this translocation has on the biologic activity of cetuximab is unknown.12
In general, cetuximab mediates its action through the inhibition of tumor proliferation, angiogenesis, and metastasis, induction of apoptosis, and/or promotion of cell-cycle arrest.1,12 It also serves as a radiosensitizer when used concurrently with traditional chemotherapeutic agents. Cetuximab promotes cell-cycle arrest in the G1 phase, which culminates in cellular apoptosis and leads to overall inhibition of tumor proliferation. In a separate signaling cascade, angiogenesis is interrupted by the inhibited production of angiogenic factors including vascular endothelial growth factor (VEGF). This antiangiogenic activity mediates a decrease in microvessel density of the tumor while advancing endothelial cell apoptosis.3 Cetuximab may also prevent nuclear transport of EGFR, thereby inhibiting DNA repair mechanisms that address chemo- or radiotherapeutically induced DNA damage.13 Finally, cetuximab likely participates in antibody-dependent cell-mediated cytotoxicity (ADCC) secondary to its IgG1 structure.4
Reported toxicities associated with cetuximab include constitutional symptoms such as fevers, chills, fatigue, and nausea, infusion reactions, and hypersensitivity reactions, as well as an acneiform skin rash, reported in some studies in up to 88% of patients.3 Severity of the rash has been linked in several studies to improved efficacy and therapeutic response.2 However, cetuximab is, overall, well tolerated. In phase I studies regarding the immunogenicity of cetuximab, fewer than 4% of patients developed HACAs following administration of the medication.1
Colorectal Cancers
Following pre-clinical evaluation of cetuximab supporting its antitumor activity, early phase I/II clinical trials were designed to evaluate the effect of cetuximab in the setting of metastatic colorectal cancers. Two early non-randomized studies demonstrated both single-agent and combination chemotherapy (with irinotecan) efficacy, with respective response rates of 11–17% and disease control rates of 46–48% in irinotecan-refractory patients.3,6 Subsequently, the Bowel Oncology with Cetuximab Antibody (BOND) phase II clinical trial randomized irinotecan-refractory patients to a regimen of cetuximab plus concurrent irinotecan versus cetuximab alone. In this study, patients undergoing combination therapy enjoyed a statistically significant response rate of 23%, a tumor control rate of 56%, a median time to progression of 4.1 months, and a median overall survival of 8.6 months. This response compared favorably with the data for the single-agent cetuximab arm, which demonstrated a response rate of 11%, control rate of 32%, median time to progression of 1.5 months, and median overall survival of 6.9 months.6 These data led the FDA in 2004 to extend approval to cetuximab for the single-agent or combination chemotherapeutic treatment of EGFR-positive, irinotecan-refractory metastatic colorectal cancer. A subsequent phase III trial demonstrated increased mean progression-free survival when cetuximab was combined with folinic acid, fluorouracil and irinotecan (FOLFIRI) in the first-line management of metastatic colorectal cancer.14
Several studies have suggested an association between severity of dermatological toxicity and treatment response to cetuximab.6 The Dose-escalation study using up to twice the standard dose of cetuximab in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST) trial was designed to evaluate the prognostic value of acneiform rash as a surrogate for treatment response in chemotherapy-refractory metastatic colorectal cancer patients.15 These patients underwent concurrent cetuximab and irinotecan treatment at typical dose schedules. After three weeks of therapy, patients were assessed for extent of skin involvement, and those not achieving grade 2 skin toxicity were randomized to maintenance of standard-dose cetuximab versus dose escalation until the appearance of grade 2 toxicities or a dose cap was reached. Comparison of the response rates revealed that patients with at least a grade 2 skin toxicity either initially or in response to dose escalation enjoyed higher rates—34 and 30%, respectively—than those patients who did not develop significant skin involvement with standard-dose cetuximab.15
Squamous Cell Cancers of the Head and Neck
Squamous cell carcinomas of the head and neck encompass several primary sites of histologically similar tumors. Approximately 50% of patients can achieve a cure with initial treatment. The most common location for recurrence is locoregional, and local recurrence is a common etiology of death in these patients.13 Traditional risk factors for these cancers include tobacco products and excessive alcohol use. More recently, as in cervical cancer carcinogenesis, high-risk human papillomavirus (HPV) subtypes have been implicated in the pathogenesis of some of these cancers. HPV-related squamous cell carcinomas of the head and neck also appear to enjoy a better overall prognosis.13 Similar to recurrent cervical cancers, recurrent head and neck cancers are palliated with single- or double-agent platinum-based chemotherapy until platinum resistance is diagnosed. Initial treatment for these cancers involves surgery with adjuvant chemoradiation, definitive chemoradiation reserving surgery as salvage, or induction chemotherapy with definitive chemoradiation.13 Furthermore, squamous cell carcinomas of the head and neck have been consistently noted to have >90% overexpression of EGFR, suggesting potential activity for cetuximab in these cancers.13
Indeed, cetuximab demonstrates efficacy when applied to squamous cell cancers of the head and neck, including enhanced radiosensitivity in vitro, and with preliminary studies confirming activity in the setting of radiotherapy for locoregionally advanced tumors.3,13 A multinational, randomized phase III trial compared high-dose radiotherapy with high-dose radiotherapy plus cetuximab in previously untreated patients with stage III/IV squamous cell cancers of the head and neck. Median survival was 49 months and median duration of locoregional control was 24.4 months in the combination arm compared, with median survival of 29.3 months and 14.9 months of locoregional control in the radiation-only arm. The combination of modalities conferred a statistically significant improvement in overall survival and duration of locoregional disease control. In addition, single-agent cetuximab was evaluated in the setting of platinum-refractory metastatic squamous cell carcinoma of the head and neck, and again the therapy demonstrated efficacy with a median response duration of 5.8 months. Armed with these efficacy data, the FDA approved cetuximab for use in squamous cell cancers of the head and neck in combination with radiotherapy for first-line treatment, and also as single-agent therapy for recurrent or metastatic disease.13
Cervical Cancer
Worldwide, cervical cancer is the second leading cause of cancer deaths in women.16,17 Although the incidence in the US has been decreasing over the past two decades secondary to the availability of effective screening programs, cervical cancer remains an important public health concern.7 In 2009, the National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) Cancer Statistics Review estimates that 11,270 women will be diagnosed with cervical cancer and 4,070 women will die from their disease.18 One-third of those patients presenting with invasive cancer will eventually succumb to their disease, despite innovations in cancer screening and prevention.17,19 Early-stage cervical cancers can be treated successfully and with equal efficacy using either radical surgery or radiation.20 However, approximately 25% of women have advanced disease at diagnosis, and the overall survival for these patients is low, even with multimodality therapies.9 Furthermore, despite improvements in treatment, up to 35% of women will eventually develop persistent, recurrent, or metastatic cervical cancers, which will respond only briefly to platinum-based chemotherapy.8,17
Historically, radiation was the mainstay of treatment for locally advanced cervical cancer.21 In 1999, five large clinical trials from the Gynecologic Oncology Group (GOG), Southwest Oncology Group (SOG), and Radiation Therapy Oncology Group (RTOG), encompassing more than 1,800 patients, published remarkably consistent data that changed the landscape of treatment of cervical cancer.11,16,22,23 These trials each demonstrated a reduction in relative risk of death of 30–50% with the addition of concurrent cisplatin chemotherapy as a radiosensitizer.11,16 These data led the NCI to endorse platinum-based chemotherapy for the treatment of locally advanced cervical cancer. Since then, single-agent cisplatin with radiation has been the mainstay of treatment, and it continues to be the most active chemotherapeutic agent in cervical cancer.17,21,23 More recently, the GOG has conducted several trials aimed at the identification of other single or multi-agent radiosensitizer regimens that demonstrate improved response and overall survival rates while maintaining satisfactory quality of life.11,16,22
Recurrent cervical cancer usually cannot be cured, particularly when surgical resection is not feasible and radiation salvage is not possible secondary to previous treatment, or when patient performance status is low.21 Thus, the goal for these patients is palliation of symptoms while preserving quality of life, and systemic chemotherapy remains the mainstay of therapy.7,16,17,19,21,24 In the early 1980s, cisplatin was first investigated for the treatment of advanced and recurrent cervical cancers with a reported response rate of 38%.19 Overall survival using cisplatin in this setting was demonstrated to be seven months.17 Since that time, various agents have been evaluated for response in the setting of recurrent or advanced disease,17,24 and of the 21 demonstrating efficacy with response rates of at least 15%, the five yielding the greatest activity include cisplatin, paclitaxel, topotecan, vinorelbine, and ifosfamide.19
Combination chemotherapy regimens have been studied by the GOG in the hope of capturing the effects of single-agent additive or synergistic activities while distributing drug toxicities.17,24 Despite multiple studies investigating the efficacies of combined regimens of agents with known activity against recurrent cervical cancer, only one GOG protocol (GOG 179) demonstrated an improvement in overall survival. The cisplatin plus topotecan arm enjoyed improved median overall survival (9.4 versus 6.5 months), median progression-free survival (4.6 versus 2.9 months), and response rates (27 versus 13%) compared with the cisplatin single-agent arm.25 In 2006, following the results of GOG protocol 179, the combination of cisplatin plus topotecan was FDA-approved for the first-line treatment of recurrent cervical cancer.7,17,21,24,25 Other investigations, intent on improving patient tolerance and quality of life measures, have explored the use of carboplatin and paclitaxel in the recurrent cervical cancer setting with promising results, although randomized head-to-head trials are lacking.21
In an effort to further improve treatment efficacy, several studies have assessed the effect of adding additional cytotoxic agents to the cisplatin-containing doublets; however, none of these so-called triplets have shown improvement over cisplatin-based mono- or doublet therapy.17,19 Given the limitations of effective traditional chemotherapy regimens for advanced and recurrent cervical cancer, focus has necessarily moved to the new frontier of biologic therapy.10
Epidermal Growth Factor Receptor and Cervical Cancer
The precise role of EGFR in cervical cancer has been an ongoing investigation.9 While early studies demonstrated overexpression in cervical cancers and cervical intraepithelial neoplasia similar to that found in other solid tumors, later research provided less convincing data, noting only a small percentage of differential expression of EGFR in malignant versus healthy cervical epithelium.9 In fact, the reported range of EGFR overexpression in cervical cancers is wide—6–85%8,10,11— and microarray analysis of 23 human cervical cancer cell lines confirmed wide variation in levels of expression of EGFR, suggesting likely differential sensitivity of these tumors to cetuximab therapy.8 Newer data also suggests that immunohistochemical analysis may not capture EGFR expression consistently, and could account for the wide variation in expression levels seen in previous studies.9 Despite early contradictory results, more recent studies confirm EGFR overexpression as a poor prognostic indicator in cervical cancers, similar to data reviewed earlier regarding other tumors.7,8,10,11 Bellone et al. tested EGFR expression in both primary and established cervical cancer cell lines, and repeatedly demonstrated higher levels of EGFR expression in the recurrent or metastatic cell lines in contrast to primary sites. This differential expression suggests a role for biologic targeting of cervical cancers in the recurrent or metastatic setting where effective salvage therapies are lacking.8 Furthermore, the reported activity of cetuximab in metastatic colorectal cancers,3,6 and more importantly its efficacy in the histologically similar squamous cell carcinomas of the head and neck,13 provided the foundation rationale for its use in cervical cancer. In particular, infection with high-risk HPV genotypes has been implicated in the development of at least 25% of squamous cell carcinomas of the head and neck,26,27 as well as the majority of cervical cancers.9,16
Cetuximab and Cervical Cancer
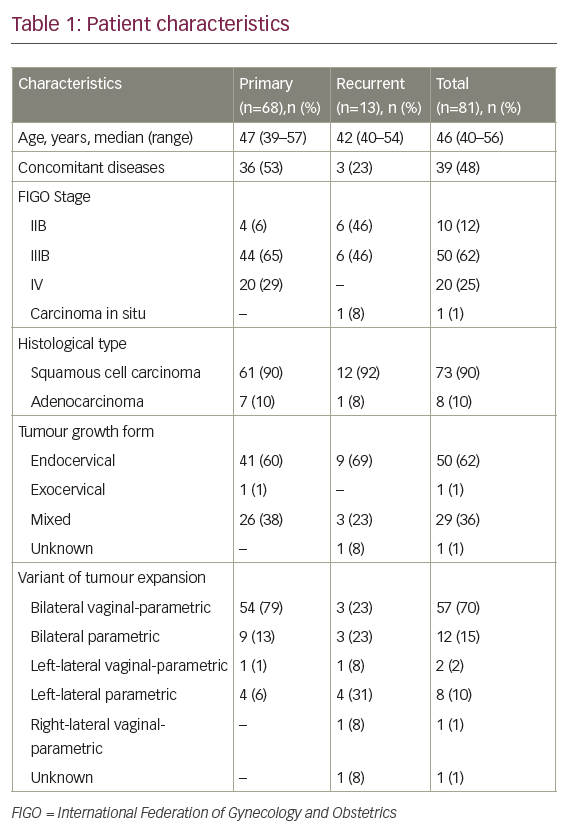
Early in vitro studies of cervical cancers with cetuximab revealed exquisite sensitivity of these tumors to monoclonal antibody therapy.8 Based on the known efficacy of cisplatin and topotecan for advanced cervical cancer, and the promising results observed for cetuximab in the treatment of colon and head and neck squamous cell carcinoma, a phase II trial was designed to investigate the efficacy of the combination regimen with the addition of cetuximab. The chemotherapeutic regimen evaluated included cisplatin 50mg/m2 on day one plus topotercan 0.75mg/m2/day from days one to three every three weeks combined with cetuximab (initial dose of 400mg/m2 followed by subsequent weekly dose of 250mg/m2). Nineteen of the planned 44 patients were enrolled prior to premature closure of the study due to higher than expected severe toxicities.7
Only two (11%) patients completed the planned six cycles of chemotherapy. Treatment was stopped for three (17%) of patients secondary to disease progression and six (33%) for toxicity. Of the 18 patients who received study treatment (one enrolled patient never started therapy), 13 had to be hospitalized for toxicity events, with a median duration of 7.8 days hospitalization.7 Five (28%) patients died during treatment: two patients died from sepsis in the setting of grade 4 neutropenia and thrombocytopenia. These deaths were clearly attributable to the treatment. One patient died from pulmonary embolism with relationship to treatment considered as ‘possible’, and another patient died from acute respiratory distress syndrome related to an inhalation pneumopathy that was considered not study related.
Grades 3 and 4 neutropenia occurred in 72% of patients, while grades 3–5 infection and febrile neutropenia occurred in 39 and 28% of patients, respectively.7 Grades 3 and 4 thrombocytopenia occurred in 61% of patients. Two (11%) patients had grades 3 or 4 renal toxicity and two (11%) patients experienced pulmonary embolism. Grade 3 skin reactions were observed in four (22%) patients. The severe hematological toxicities notwithstanding, an overall response rate of 32% was demonstrated despite the abbreviated study interval, indicating significant activity of the cetuximab-containing regimen.7 The median progression-free and overall survival observed were 5.7 and 7.3 months, respectively.
Several GOG studies regarding the use of cetuximab for the treatment of cervical cancers are ongoing, including a study of the efficacy of single-agent cetuximab in recurrent cervical cancer, a phase I trial of tailored radiation therapy with concomitant cetuximab and cisplatin as radiosensitizers in primary cervical cancer, and a phase II trial comparing cetuximab combined with cisplatin for recurrent or persistent cervical cancers.10,28 Preliminary data from the phase II trial evaluating the addition of cetuximab to cisplatin for the treatment of advanced, recurrent and previously treated cancers of the cervix reveals that the combination regimen is well tolerated, with minimal toxicity. Seventy-six patients were entered into the study. Of these, 69 were eligible and evaluable. Fifty-six (81%) patients had received prior radiation. Forty patients (58%) had previously received chemotherapy. There were eight (17.2%) responses, and a 25% progression-free survival at six months was observed. EGFR assessment in the tumors of enrolled patients confirmed immunohistochemical overexpression of EGFR protein in this malignancy. EGFR was expressed in 98% of tumors analyzed. Of the 48 tumors analyzed, 25 (52%) had EGFR expression in 81% of cells (high percent positive). An increased EGFR expression was not notably associated with age, performance status, number of prior regimens, or having tumor response or stable disease. Furthermore, higher levels of EGFR overexpression conferred worse prognosis in terms of increased likelihood of disease progression at six months, as well as decreased responsiveness to cetuximab. Pre-treatment EGFR expression in ≥81% of cells, however, was associated with a twofold higher risk of progression (hazard ratio [HR] 1.99, 95% confidence interval [CI] 1.08–3.68), but not overall survival (HR 1.37, 95% CI 0.72–2.61). The combination of cisplatin and cetuximab therapy demonstrated rates of disease stabilization and clinical objective response comparable to those seen with the agents with highest activity in the recurrent setting.28
Resistance to Epidermal Growth Factor Receptor Modulation
While modulation of EGFR by targeted therapies such as cetuximab has demonstrated efficacy against various cancers in phase I/II trials, a subset of patients with these diseases have reduced or absent initial responsiveness to the therapy, and others subsequently develop therapeutic resistance.5 Furthermore, several investigators have demonstrated that density of EGFR expression in tissue does not correlate with response to anti-EGFR therapy.6 Several potential etiologies of the decreased efficacy of anti-EGFR therapy exist, including somatic mutations of the EGFR, circumvention of the EGFR by preferential activation of neighboring TKRs, and EGFR-independent activation of intracellular signaling intermediaries such as v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), and others.5 In fact, mutations of the KRAS oncogene have been identified in 15–30% of patients with non-small cell lung cancers and 40–45% of patients with colorectal cancers. These mutations result in constitutive activation of the KRAS-dependent pathway independent of EGFR blockade, and necessarily confer resistance to cetuximab.3,5
Conclusion
In the setting of advanced and recurrent cervical cancers, traditional therapies with the best efficacy still provide response rates of only 17–38%. The search for novel therapeutics to address this relative lack of efficacy is ongoing, particularly in the realm of targeted drug therapy, with its rational mechanisms of action and complementary toxicities. The combination of cetuximab with cisplatin is adequately tolerated and appears to increase progression-free survival beyond cisplatin therapy. Patients with high EGFR expression status, however, are less likely to remain progression-free of disease at six months. Stratification of patients based upon EGFR status may select a group for whom this regimen is most effective. ■