Human epidermal growth factor receptor 2 (HER2) is encoded by a proto-oncogene with important roles in the promotion of cell proliferation, differentiation and angiogenesis via the activation of Phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) downstream signalling after it forms homodimers or heterodimers with other HER family members.1–2 HER2 overexpression, mainly caused by gene amplification, allows HER2 activation, even in the absence of ligand binding.3
HER2 amplification is the most frequently observed in breast cancer; accordingly, the status of HER2-targeted therapy is the most advanced in this cancer type. Although HER2 overexpression is a poor prognostic factor in breast cancer, HER2-targeted therapy has improved clinical outcomes dramatically.4–5 A variety of agents – including trastuzumab, pertuzumab, lapatinib, ado-trastuzumab emtansine (T-DM1) and neratinib – have been approved for HER2-positive breast cancer. Recently, novel anti-HER2 agents – including trastuzumab deruxtecan (T-DXd), tucatinib and margetuximab – have demonstrated significant improvements in clinical outcome.6–8 T-DXd is an antibody–drug conjugate composed of an anti-HER2 antibody, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor.9 T-DXd has shown a remarkable efficacy in DESTINY-Breast01 (A study of DS-8201a in metastatic breast cancer previously treated with trastuzumab emtansine [T-DM1]; ClinicalTrials.gov identifier: NCT03248492), a multicentre, single-arm, phase II study including 184 patients with HER2-positive breast cancer who previously received HER2-targeted therapies, leading to US Food and Drug Administration approval.6
Recent genotyping studies have revealed HER2 amplification in multiple cancer types. In light of the successes with trastuzumab and other agents in with HER2-positive breast cancer, interest has emerged in exploring the efficacy of HER2-targeted therapies for other cancer types. Specifically, gastrointestinal (GI) cancer is the second-most common cancer with HER2 amplification. Multiple HER2-targeted therapies have been developed and are in clinical trials. In this review, we focus on recent developments in HER2-targeted therapies and future perspectives for using them to treat GI cancers, including gastric cancer, colorectal cancer, oesophageal cancer, biliary tract cancer and pancreatic cancer.
Gastroesophageal junction cancer
Among GI cancers, the greatest advances in HER2-targeted therapy have been achieved for gastroesophageal junction (GEJ) cancers. The frequency of HER2 amplification in gastric cancers is 15–25% and is higher than the overall frequency in GI cancer.10–13 In particular, GEJ cancers have a higher rate of HER2 amplification (32.2%) than those of gastric cancers (21.4%); and intestinal tumours also have a higher rate of HER2 amplification (31.8%) than those with a diffuse type (6.1%).13
A distinct characteristic of HER2-positive gastric cancer, including GEJ cancer, that differentiates it from HER2-positive breast cancer is intra-tumoural heterogeneity,14–15 which is characterised as the presence of areas with different HER2 immunohistochemistry (IHC) scores within the same tumour. Moreover, the criteria for HER2 positivity differ slightly between gastric cancer and breast cancer. In the ToGA trial, which was the first randomised phase III study of HER2-positive gastric or GEJ cancer, HER2 positivity was defined as either: (1) IHC 3+ (defined as moderate to strong, complete or basolateral membrane staining in >10% of tumour cells); or (2) in situ hybridisation (ISH)-positive (defined as a HER2:CEP17 ratio of >2).16 Based on these criteria, 22.1% of tumours were classified as HER2 positive. However, because the IHC 1+/0 and the ISH-positive subgroup did not show a clinical benefit in this trial, HER2 positivity has since been redefined as IHC 3+ or IHC 2+ and ISH-positive tumours in clinical settings.17
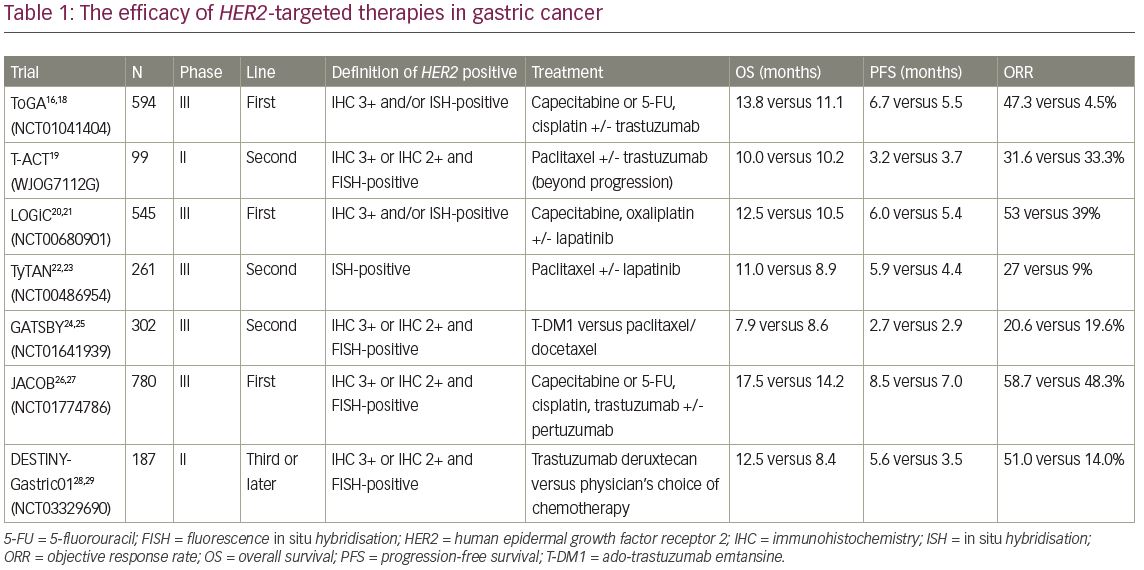
In the ToGA trial (A study of herceptin [trastuzumab] in combination with chemotherapy compared with chemotherapy alone in patients with HER2-positive advanced gastric cancer; ClinicalTrials.gov Identifier: NCT01041404), 584 patients with HER2-positive gastric or GEJ cancer were recruited according to the above criteria and were randomised to receive fluoropyrimidine and cisplatin with trastuzumab (monoclonal antibody targeting HER2) or placebo.16,18 In the trastuzumab group, median overall survival (OS) was 13.8 months and median progression-free survival (PFS) was 6.7 months. These durations were significantly longer than those in the placebo group (i.e., 11.1 and 5.5 months, respectively) (Table 1).16,18–29 In particular, in patients who were IHC 3+ or 2+ and fluorescence in situ hybridisation (FISH)-positive, trastuzumab with chemotherapy demonstrated remarkable efficacy, with a median OS of 16.0 months (hazard ratio [HR] 0.65, 95% confidence interval [CI] 0.51–0.83), whereas the survival benefit was not observed (HR 1.07, 95% CI 0.70–1.62) in patients with low levels of HER2 protein (IHC 0/1+ and FISH-positive). According to the survival benefit in patients with high HER2 expression, IHC 3+ or IHC 2+ and ISH-positive disease is considered positive for gastric cancer.
On the other hand, the efficacy of trastuzumab beyond disease progression was not confirmed in gastric cancer. In the T-ACT (Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer [WJOG7112G]) randomised phase II trial, 91 patients with HER2-positive gastric or GEJ cancer with disease progression after first-line chemotherapy plus trastuzumab, were assigned to groups treated with paclitaxel with or without trastuzumab.19 The median OS and PFS were 10.0 and 3.2 months in the trastuzumab combination group and 10.2 and 3.7 months in the paclitaxel monotherapy group (OS: HR 1.23, p=0.20; PFS 0.91, p=0.33), which was not significant (Table 1). On exploratory analyses, HER2 expression was lost after first-line chemotherapy in 11 of 16 (69%) patients whose tumour tissues were available, which might cause no clinical benefit by trastuzumab beyond disease progression.
Lapatinib, a tyrosine kinase inhibitor (TKI) that binds to the intracellular tyrosine kinase domains of epidermal growth factor receptor (EGFR) and HER2, blocking autophosphorylation and downstream signalling, has been investigated for gastric cancer. In the TRIO-013/LOGiC trial (Lapatinib optimization study in ErbB2 [HER2] positive gastric cancer: a phase III global, blinded study designed to evaluate clinical endpoints and safety of chemotherapy plus lapatinib; ClinicalTrials.gov Identifier: NCT00680901), 545 patients with gastric or GEJ cancer harbouring HER2 amplifications, defined as IHC 3+ or ISH-positive regardless of HER2 IHC score, were assigned to groups receiving capecitabine and oxaliplatin plus either lapatinib or placebo in the first-line setting.20,21 The primary endpoint, median OS, was 12.2 months in the lapatinib arm and 10.5 months in the placebo arm, which was not significant (HR, 0.91, p=0.349) (Table 1). In a pre-planned exploratory subgroup analysis, OS benefit was suggested in patients under 60 years of age and those of Asian ethnicity receiving lapatinib. On the other hand, no statistically significant correlations were observed between HER2 IHC status and survival.
Tykerb with taxol in Asian HER2-positive gastric cancer (TyTan; ClinicalTrials.gov Identifier: NCT00486954) is a phase III trial involving 261 patients with HER2 amplification-positive gastric or GEJ cancer regardless of HER2 IHC score, previously treated with trastuzumab.22,23 Enrolled patients were randomised to receive paclitaxel with or without lapatinib as the second-line chemotherapy. The median OS was 11.0 months for lapatinib plus paclitaxel versus 8.9 months for paclitaxel alone with no significant difference (p=0.104) (Table 1). However, lapatinib is not approved for gastric cancer because neither the LOGiC nor TyTan trials showed significant improvement in OS in the intent-to-treat population. The lack of survival benefit in the LoGiC and TyTan may be attributed to inclusion of patients with low HER2 expression, which was suggested not to have clinical efficacy by HER2-targeted therapy based on results of the ToGA study. Indeed, in a subgroup analysis of the TyTan trial, patients with IHC 3+ (101/192, 53%) in the lapatinib plus paclitaxel group had better OS (14.0 months versus 7.6 months) and PFS (5.6 months versus 4.2 months) than those in the paclitaxel-only group. The LoGiC trial also included 81 (17%) patients with HER2 IHC 0/1+ disease, though this trial did not show significant OS improvement even in patients with IHC 2+/3+ disease. These results indicated that HER2-targeted therapy needs to be developed for gastric cancer with high HER2 expression.
T-DM1 is an antibody–drug conjugate composed of trastuzumab, a thioether linker, and the potent microtubule inhibitor, DM1. In the GATSBY study (A study of trastuzumab emtansine versus taxane in participants with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer; ClinicalTrials.gov Identifier: NCT01641939), an open-label, adaptive phase II/III trial, the efficacy of T-DM1 was evaluated in comparison with taxane for patients with previously treated HER2-positive metastatic gastric or GEJ cancer.24,25 In this study, patients who were IHC 3+ or 2+ and FISH-positive were eligible. Seventy-seven per cent of enrolled patients had received HER2-targeted therapy as first-line chemotherapy (almost all containing trastuzumab), and were randomly assigned to receive either T-DM1 (2.4 mg/kg weekly) or taxane (2:1). OS was not improved in the T-DM1 group compared with the taxane group (median 7.9 versus 8.6 months). Furthermore, median PFS was also not improved (2.7 versus 2.9 months) (Table 1). Although T-DM1 has previously shown activity in patients with HER2-positive metastatic breast cancer who had progressed during or after HER2-targeted therapy, GATSBY could not show clinical benefit in HER2-postive gastric cancer compared with standard chemotherapy.30 One possible reason for this discrepancy is that emtansine might be less active in gastric cancer; vinca alkaloids, which inhibit microtubule polymerisation, seem to be less active in gastric cancer than in other cancers. Another possible reason is alteration of HER2 status after first-line chemotherapy with trastuzumab. Although most patients were selected on the basis of archival HER2 status in this study, there is evidence that HER2 status might be altered during progression on trastuzumab because of changes in the molecular profile of the tumour.31 In addition, HER2 overexpression has a higher incidence of intra-tumoural heterogeneity in gastric cancer compared with breast cancer. The focality of HER2 overexpression might affect the activity of T-DM1 because it only targets HER2-overexpressed cancer cells without bystander effect for surrounding HER2 negative cells due to non-cleavable linker.
Pertuzumab is a humanised monoclonal antibody, which binds a different epitope than the one bound by trastuzumab, that inhibits HER2 heterodimerisation. The CLEOPATRA study (A study to evaluate pertuzumab + trastuzumab + docetaxel vs. placebo + trastuzumab + docetaxel in previously untreated HER2-positive metastatic breast cancer; ClinicalTrials.gov Identifier: NCT00567190) has demonstrated that the addition of pertuzumab to trastuzumab plus docetaxel for HER2-positive breast cancer can improve clinical outcomes.32,33 In gastric cancer, the efficacy of pertuzumab was investigated in the JACOB study (A study of pertuzumab in combination with trastuzumab and chemotherapy in participants with human epidermal growth factor receptor 2 (HER2)-positive metastatic gastroesophageal junction or gastric cancer; ClinicalTrials.gov Identifier: NCT01774786), a randomised phase III trial to evaluate the efficacy of the addition of pertuzumab to trastuzumab plus capecitabine or 5-fluorouracil and cisplatin for patients with metastatic HER2-positive gastric or GEJ cancer.26,27 In this study, HER2 positivity criteria was same as that of GATSBY trial (IHC 3+ or 2+ and FISH-positive). The median PFS was longer in the pertuzumab group than in the control group (8.5 versus 7.0 months; p=0.0001), and objective response rate (ORR) also improved (56.7 versus 48.3%; p=0.026). The primary endpoint, OS, in the pertuzumab group tended to be better than that of the experimental group (17.5 versus 14.2 months), but the difference was not statistically significant (HR 0.84, p=0.057) (Table 1). It was difficult to assess which patients might be more likely to benefit from pertuzumab, because the HR for OS was consistent across patient subgroups. The relative dose intensity of chemotherapy treatment was numerically reduced in the pertuzumab group compared with the placebo group because of increasing of grade 3 or worse adverse events such as diarrhoea. This might also have played a part in the study outcome.
T-DXd, as described above, is an antibody–drug conjugate agent recently approved for breast cancer. Among patients in a phase Ib study, those with HER2-positive gastric or GEJ cancer post-trastuzumab were assigned in part IIb. Tumour HER2 status was assessed using archival samples. A total of 44 patients with HER2-positive gastric or GEJ cancer received 5.4 or 6.4 mg/kg T-DXd. Finally, 19 of 44 patients (43.2%) achieved an objective response, with 35 of 44 (79.5%) achieving disease control. After a median follow-up of 5.5 months, the median OS and PFS were 12.8 and 5.6 months, respectively (Table 1).28 Based on these promising results, the phase II DESTINY-Gastric01 trial (DS-8201a in human epidermal growth factor receptor 2 (HER2)-expressing gastric cancer; ClinicalTrials.gov Identifier: NCT03329690), for patients with previously treated HER2-positive (IHC 3+ or 2+ and FISH-positive) metastatic gastric or GEJ cancer, was performed.28,29 The results were reported recently; OS was longer with T-DXd than with the physician’s choice of chemotherapy (median, 12.5 versus 8.4 months; p=0.01) and ORR in T-DXd was higher than that of chemotherapy (51 versus 14%, p<0.001).28 Accordingly, the approval of this drug for clinical use is anticipated in Japan, where the SAKIGAKE designation system to promote driving early practical application for innovative pharmaceutical products has been granted for this indication.
Colorectal cancer
The incidence of HER2 amplification is 1–5% in unselected colorectal cancer (CRC).34–37 HER2 amplification is more frequent in CRC harbouring wild-type RAS/BRAF (5–8%) than in CRC harbouring mutant RAS/BRAF. HER2 is not only an oncogenic driver, but also is associated with poor efficacy of anti-EGFR antibody treatment, based on preclinical and clinical data.38–40
A preclinical, multi-armed, Italian study, using large xenograft cohorts from 85 patient-derived, genetically characterised metastatic CRC (mCRC) samples (‘xenopatients’) revealed that combining the anti-HER2 agents trastuzumab and lapatinib induced overt, long-lasting tumour regression in HER2-amplified xenopatients, although each drug alone was not effective.40 Based on these data, the efficacy of dual HER2-targeted therapy was evaluated in the HERACLES trial (Evaluation of trastuzumab in combination with lapatinib or pertuzumab in combination with trastuzumab-emtansine to treat patients with HER2-positive metastatic colorectal cancer; ClinicalTrials.gov Identifier: NCT03225937), a multicentre, open-label phase II trial for patients with HER2-positive mCRC and wild-type KRAS exon 2 after standard chemotherapy and anti EGFR therapy.35,41 In this study, HER2 positivity was defined as: (1) IHC 3+ (membrane staining in ≥50% of tumour cells); or (2) IHC 2+ and FISH-positive. Among 914 patients with tumours harbouring wild-type KRAS exon 2, 48 patients (5.3%) were identified as HER2 positive.35 A total of 27 patients were enrolled and received trastuzumab and lapatinib therapy. Eight of 27 patients achieved an objective response, including a complete response in one patient. The ORR was 30%, the disease control rate (DCR) was 74%, and median OS was 46 weeks (Table 2).35,41–52 Although this was a small, non-randomised, phase II trial, dual HER2-targeted therapy can be expected as a new option for patients with HER2-positive mCRC on the basis of these favourable results.
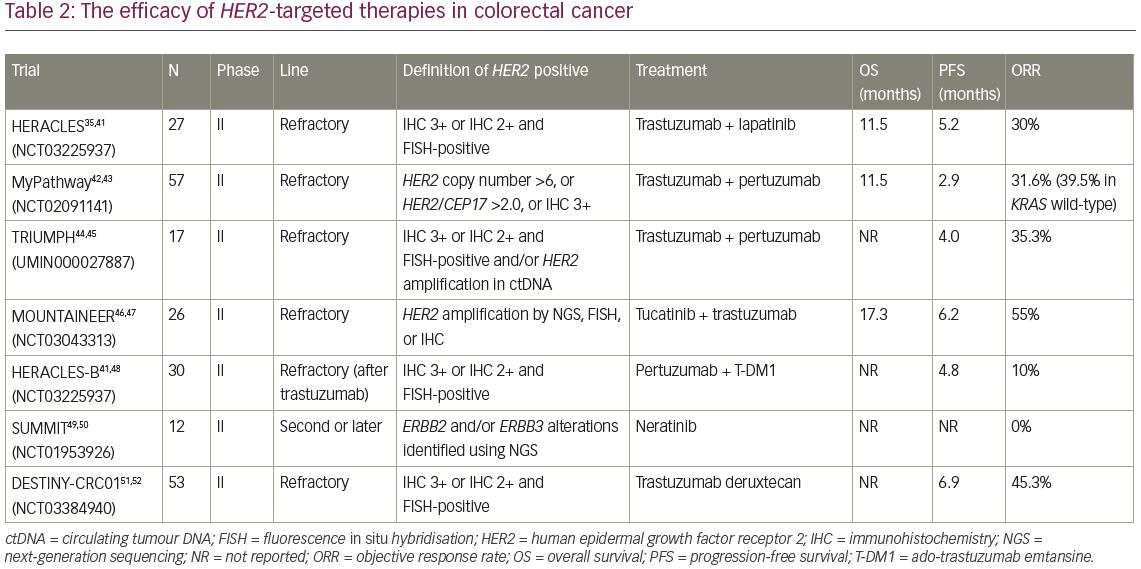
Subsequently, combination therapy with trastuzumab and pertuzumab was also evaluated in MyPathway, an ongoing phase II basket trial for patients with advanced solid tumours harbouring specific genetic or molecular alterations.42 In this trial, patients with HER2-positive solid tumours received treatment with trastuzumab and pertuzumab. For eligibility, HER2 positivity was defined as HER2 copy number >6 by next-generation sequencing (NGS) or ISH, HER2/CEP17 ratio >2.0 by FISH, or IHC 3+. A total of 34 patients with HER2-amplified mCRC were enrolled. At a median follow-up of 7.3 months, the ORR was 31.6% (18/57) and the median duration of response was 5.9 months (Table 2). Interestingly, in the wild-type KRAS group only, ORR reached about 40%, which was statistically higher than the ORR (12.5%) of the mutant KRAS group in this study.
The phase II TRIUMPH study (Multicenter phase II study to evaluate efficacy and safety of combination therapy with trastuzumab and pertuzumab in patients with HER2-positive metastatic colorectal cancer; Clinical trial identification: UMIN000027887) also evaluated combination therapy with trastuzumab and pertuzumab for patients with HER2-positive mCRC.44 Patients with wild-type RAS harbouring HER2-positive mCRC confirmed by IHC/FISH (tissue-positive group) and/or HER2 amplification by a circulating tumour DNA (ctDNA) analysis using Guardant360 assay (ctDNA-positive group), were enrolled.44 A total of 17 patients received the HER2-targeted therapy with trastuzumab plus pertuzumab every 3 weeks. In the tissue-positive group, six of 17 patients (35.3%) achieved objective responses, including one complete response (Table 2). In the ctDNA-positive group, five of 15 patients (33.3%) achieved an objective response, including one complete response. In an exploratory analysis, five of 11 patients (54.5%) with wild-type RAS/BRAF/PIK3CA/HER2 by ctDNA analysis at baseline, achieved a confirmed objective response, while none of the five patients with any mutation in any of the four genes at baseline had an objective response. Furthermore, the IHC scores for all patients who achieved an objective response were 3+, while none of the patients with IHC 2+ had a clinical response. Currently, the S1613 study (Trastuzumab and pertuzumab or cetuximab and irinotecan hydrochloride in treating patients with locally advanced or metastatic HER2/Neu amplified colorectal cancer that cannot be removed by surgery; ClinicalTrials.gov Identifier: NCT03365882), a randomised phase II trial to compare the efficacy of trastuzumab and pertuzumab to that of cetuximab and irinotecan for mCRC with HER2 amplification, is ongoing.53,54
The efficacy of tucatinib, which is a highly selective oral small molecule TKI of HER2, was evaluated in the MOUNTAINEER study (Tucatinib plus trastuzumab in patients with HER2+ colorectal cancer; ClinicalTrials.gov Identifier: NCT03043313).46,47 This was a single-arm phase II trial for patients with HER2-positive, wild-type RAS mCRC who had received standard chemotherapy including an anti-vascular endothelial growth factor (VEGF) monoclonal antibody. A total of 26 patients were enrolled and received combination therapy with tucatinib and trastuzumab. Among 22 patients who completed ≥1 evaluation, 12 (55%) achieved an objective response and an additional two showed stable disease over 4 months. The median duration of response was not reached, median OS was 17.3 months, and median PFS was 6.2 months (Table 2).
HERACLES-B (HER2 amplification for colorectal cancer enhanced stratification, cohort B; NCT03225937) is an open-label phase II trial to evaluate the combination of pertuzumab and T-DM1 in untreated wild type RAS/BRAF and HER2-positive mCRC (n=30 patients).48 The ORR was 10% and DCR was 80%, with a median PFS of 4.8 months. The primary endpoints, ORR and PFS, were not met (Table 2).
In the SUMMIT study (Neratinib HER mutation basket study; ClinicalTrials.gov identifier NCT01953926), a phase II basket study for HER2-mutant solid tumours, 12 patients with mCRC harbouring HER2 mutation received treatment with neratinib, an irreversible pan-HER TKI.49,50 However, objective response was not observed. Now, the comparison of combination therapy with neratinib plus trastuzumab or neratinib plus cetuximab in patients with KRAS/NRAS/BRAF/PIK3CA wild-type mCRC is being evaluated in a randomised phase II study (ClinicalTrials.gov identifier: NCT03457896).55
In the DESTINY-CRC01 trial, a phase II, multicentre, open-label study of T-DXd in patients with HER2-expressing mCRC, a total of 78 patients received T-DXd. They received T-DXd 6.4 mg/kg every 3 weeks in three cohorts (A: HER2 IHC 3+ or IHC 2+/ISH+; B: IHC 2+/ISH-; C. IHC 1+). The primary endpoint was confirmed ORR. In cohort A (n=53), the ORR was 45.3% (24/53 pts; 95% CI, 31.6–59.6%) including 1 complete response and 23 partial responses. The median PFS was 6.0 months (95% CI, 4.1 months – not evaluated); median OS was not reached. No responses were observed in cohorts B (n=7) or C (n=18).51,52
In summary, some clinical trials for HER2-positive CRC have shown promising outcomes. However, these trials were performed based on different inclusion criteria and included small number of patients (Table 2). Since the different inclusion criteria make it difficult to interpret the efficacy of HER2-targeted therapy across each trial, an establishment of integrated HER2-positivity criteria is required in CRC.
Oesophageal cancer
There are two major pathological subtypes of oesophageal cancer, oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC) including GEJ cancer, such as Barrett’s adenocarcinoma. The frequency of HER2 amplification is high in OAC, with estimates of approximately 30% in the Cancer Genome Atlas and 32.2% in the ToGA study (in GEJ cancer).13,56 Trastuzumab plus chemotherapy is recommended in patients with HER2-positive OAC based on the results of ToGA trial.57 In OSCC, the frequency of HER2 amplification is much lower than that observed for OAC. In a genomic landscape study in Japan, HER2 amplification was detected in only 2.3% of OSCC cases.58 Another study reported that the frequency of HER2 amplification in OSCC is 3.9% (3/76), despite a high HER2 amplification rate (24.0%; 12/50) in GEJ cancer.59
In a preclinical study of HER2– and EGFR-overexpressing oesophageal cancer cell lines, trastuzumab and lapatinib inhibited cell growth and enhanced antibody-dependent cytotoxicity.60 However, prospective trials of HER2-positive OSCC are lacking to date.
Biliary tract cancer
In biliary tract cancer (BTC), HER2-targeted therapy has received attention owing to its high frequency of HER2 overexpression. In one study, 54.3% of BTC cases exhibited HER2 IHC staining (IHC score ≥1+), and 10.9% of BTC cases had an IHC score of 3+, although the reported frequency of HER2 amplification is 5–15% in BTC, particularly in gallbladder cancer.61–64 In the Nationwide Cancer Genome Screening Project in Japan, for metastatic BTC (mBTC) samples analysed by NGS, HER2 amplification was detected in three of 92 tumours (3.3%).65 Another comprehensive genomic profiling study of 260 BTCs demonstrated that HER2 mutation was identified in 4–5%, and was mutually exclusive to RAS, BRAF and NF1 mutations.66
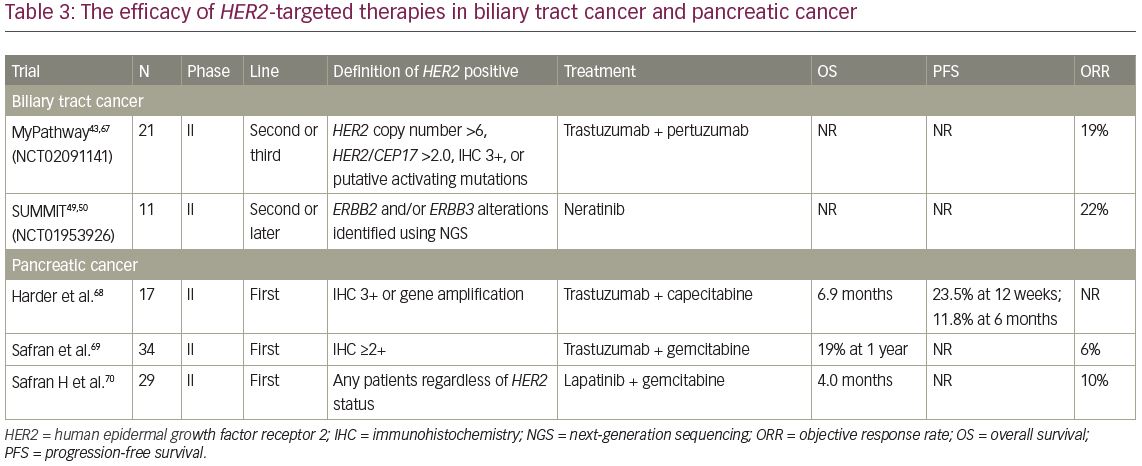
In the BTC cohort in the MyPathway trial, eight patients with HER2 amplification or HER2 overexpression and three with HER2 mutations (D277Y,f S310F, and A775-G776insYVMA) received combination therapy with trastuzumab and pertuzumab.67 At a median follow-up of 4.2 months, four patients achieved an objective response (three of eight HER2-amplified/overexpressed patients) and three had stable disease for 4 months (Table 3).43,49,50,67–70 In the SUMMIT basket study, 11 patients with mBTC harbouring HER2 mutations received treatment with neratinib, and partial responses were observed in 22% of the patients.39 In addition, the MOSCATO study, a prospective molecular profiling study for advanced cancers, reported that a complete response with trastuzumab plus chemotherapy was achieved in one patient with HER2-positive mBTC.71,72 Although sample size of these trials was small, these results suggest that HER2-targeted therapy may also be effective in patients with mBTC harbouring HER2 amplification and HER2-activated alterations.
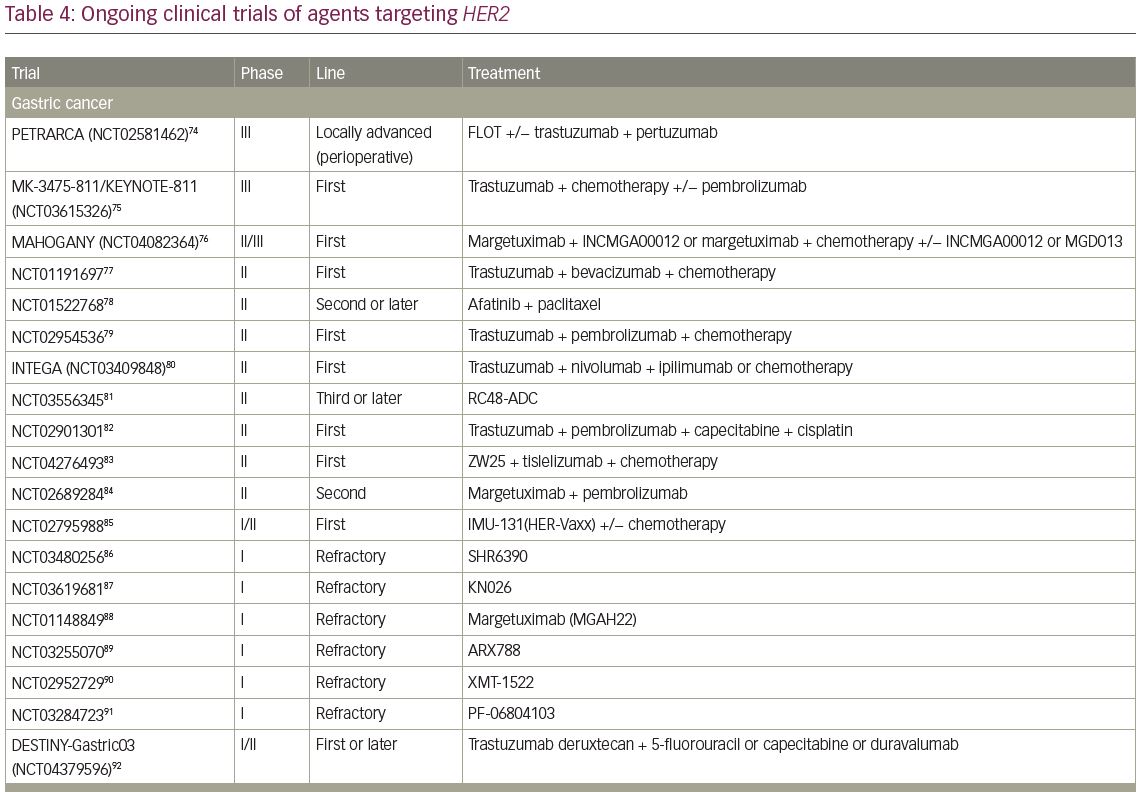
A single-arm, phase II trial of T-DXd for HER2-positive BTC, the HERB study, is ongoing in Japan (JMA-IIA00423).73 In the main cohort, IHC 3+ or IHC 2+ and ISH-positive patients determined by central imaging review are eligible. In an additional exploratory cohort, IHC 2+ and ISH-negative patients and IHC 1+ patients are eligible. The primary endpoint of this trial is ORR in the main cohort and the key secondary endpoints are PFS, OS, and ORR in patients with low HER2 expression (Table 4).54,55,73–112
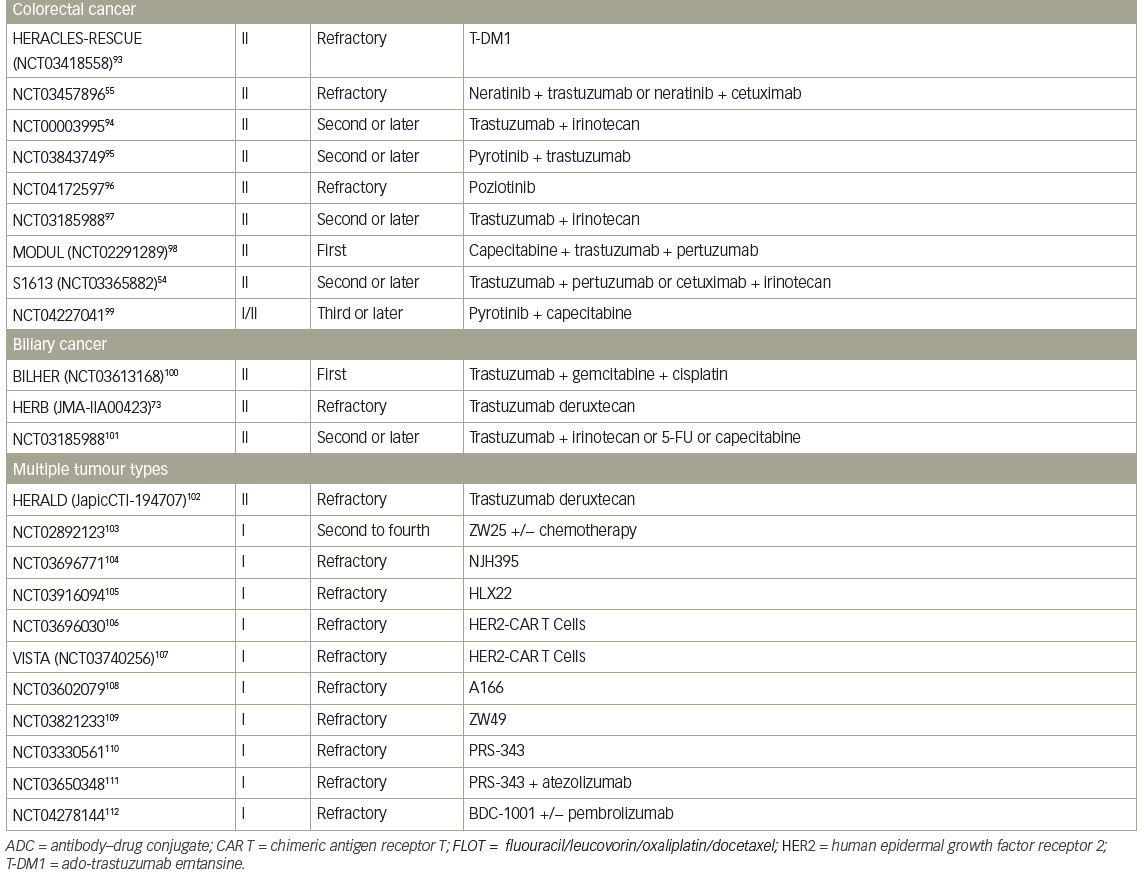
Pancreatic cancer
The frequency of HER2 overexpression has been reported to be 17–45% pancreatic cancer,113,114 with the frequency of HER2 amplification approximately 3%.113 In both pancreatic-cancer cell lines and a xenograft mouse model, HER2–targeted therapy with trastuzumab showed favourite results.115 Therefore, several trials have been performed to clarify the clinical significance of HER2 expression and amplification in patients with metastatic pancreatic cancer and to determine the potential of HER2 as a therapeutic target.
To investigate the efficacy and safety of trastuzumab plus capecitabine as first-line treatment in advanced pancreatic cancer, a prospective, single-arm, open-label, multicentre phase II trial was conducted.68 A total of 207 patients were assessed for HER2 expression and gene amplification in tumour specimens, with 22 and 31 patients diagnosed as HER2 IHC 3+ and 2+ or HER2 amplification, respectively. Among them, 17 IHC 3+ or IHC 2+ and ISH-positive patients were enrolled in the study, receiving treatment with trastuzumab and capecitabine. The rate of PFS at 12 weeks was 23.5%, and median OS was 6.9 months. These results were not an improvement over those of standard chemotherapy (Table 3).
In another phase II study of trastuzumab and gemcitabine among 34 patients with metastatic pancreatic cancer, four (12%) had IHC 3+ and 30 (88%) had IHC 2+.69 Confirmed partial responses were observed in two patients (6%) and 13 (41%) had either an unconfirmed partial response or a >50% reduction in CA 19-9. The median OS for all 34 patients was 7 months, with a 1-year survival rate of 19% (Table 3). Because these results were similar to those for gemcitabine alone, the addition of the anti-HER2 agent does not improve clinical outcomes in pancreatic cancer.
Future perspectives
The first ToGA trials demonstrated the survival benefit of HER2–targeted therapy for patients with gastric or GEJ cancer with HER2-high expression (IHC 3+ or 2+ and FISH-positive). In the HERACLES trial for HER2-positive mCRC, response rate was higher in patients with IHC 3+ compared with IHC 2+ (44% versus 14%) and high HER2 copy number was also related to better treatment outcome. These results suggest that both HER2 amplification and HER2 overexpression are important for selection of patients with GI cancer who may benefit from HER2-targeted therapy. Genomic profiling of GI cancers has revealed HER2 somatic mutation is occurring in the absence of amplification. It is unclear whether anti-HER2 antibodies are effective for tumours with HER2 mutation because preclinical studies showed trastuzumab can block cell proliferation and survival in tumour with HER2 mutation,116,117 but other studies have shown HER2 mutation weakened the inhibition of trastuzumab.118,119 Nevertheless, HER2 somatic mutation is also regarded as a treatment target in some clinical trials because small-molecule inhibitors, such as neratinib, have shown higher effectiveness in blocking the survival of cells expressing HER2 mutants in preclinical studies.116 In the SUMMIT study for solid tumour with HER2 mutation, neratinib showed promising tumour responses in particular cancer types, such as BTC.
As we have reviewed above, multiple regimens have been evaluated in GI cancers, but only limited agents have shown clinical benefits due to some potential limitations. First, the frequency of HER2 amplification is normally <10% in GI cancer; except for gastric cancer.34,58,65,113 To address this issue, broad screening using NGS and several basket trials, such as MyPathway, have recently been applied.42,67 In Japan, a nationwide cancer genome screening project, GI-SCREEN, has been in place since 2015, with more than 5,000 genome sequencing results from GI cancer tissue samples generated to date.120
In addition, ctDNA analysis, a liquid biopsy technology, may be beneficial in a clinical setting given its minimal invasiveness and short turnaround time.121,122 In a validation study of 58 patients with metastatic breast cancer, positive and negative predictive value of HER2 amplification in plasma were 70% and 92%, respectively.123 However, in metastatic GI cancer, there are reports of the relationship between HER2 copy number in ctDNA and HER2 overexpression in tissue. An analysis of 60 patients with resectable gastric cancer showed the preoperative plasma HER2 amplification correlated with the tumour HER2 amplification and overexpression (p<0.001), and sensitivity and specificity were 73.3% and 93.3%, respectively.124 In addition, HER2 copy number in ctDNA has been reported to be associated with the efficacy of HER2-targeted therapy in gastric cancer and mCRC.125,126 Therefore, ctDNA HER2 analysis may be useful for screening to identify patients with HER2 amplification who may benefit from HER2-targeted therapy.
Based on the GI-SCREEN platform, the GOZILA study (Research on liquid biopsy in patients with gastrointestinal and abdominal malignancies, including colorectal cancer; UMIN000029315) is currently in evaluating ctDNA cancer-related genome sequences using Guardant360 for more than 2,000 patients with advanced GI cancer.127,128 Patients can be enrolled in several clinical trials based on the gene alterations identified in GOZILA. For example, HERALD (A basket trial of DS-8201a, a novel HER2-targeted antibody-drug conjugate, for HER2 amplified solid tumors identified by ctDNA analysis; JAPIC ID: JapicCTI-194707) is a basket trial of T-DXd for ctDNA HER2-positive solid tumours, excluding breast, lung, gastric, colorectal, and BTC, based on the GOZILA platform.102 Second, a definition of HER2 positivity associated with treatment efficacy has yet to be established for GI cancer, except for gastric cancer. Although established criteria will likely be helpful to improve protocols and interpret results; in the past, clinical trials have been performed with different inclusion criteria by different HER2 testing modalities, such as IHC, ISH, NGS, and ctDNA (Table 2). In a recent study spanning Japan, Korea and the USA, the international diagnostic criteria for HER2-amplified mCRC have been harmonised by investigation of the relationship between HER2-IHC/FISH results and HER2 copy number determined by NGS.129 In this study, criteria using NGS as well as IHC/FISH have been proposed to identify HER2-positive CRC, which will be validated with additional clinical data in the future.
Currently, several trials involving novel HER2-targeted agents are ongoing in GI cancer (Table 4). A phase III trial investigating the efficacy of pembrolizumab, an anti-programmed cell death protein 1 (PD-1) monoclonal antibody, versus placebo in combination with trastuzumab plus chemotherapy in patients with chemotherapy-naive HER2-positive gastric or GEJ cancer is currently ongoing (KEYNOTE-811; ClinicalTrials.gov identifier: NCT03615326).75 This is based on the promising results of a phase II trial (ORR of 87%), which reported a median PFS of 11.4 months, with a 12-month OS rate of 76%.130
Margetuximab is an anti-HER2 antibody with increased affinity for both the low-affinity and high-affinity forms of CD16A, an Fc receptor. In a first-in-human phase I study, 66 patients with HER2-positive breast, gastric, or other cancers received margetuximab at doses of 0.1–6.0 mg/kg for 3 of every 4 weeks or once every 3 weeks (10–18 mg/kg).131 Among 60 response-evaluable patients, confirmed partial responses were observed in seven (12%) patients, including two with gastroesophageal cancer, and stable disease was observed in 30 (50%) patients. An ongoing phase Ib/II study is investigating the effects of margetuximab in combination with pembrolizumab in patients with HER2-positive gastric or GEJ cancers previously treated with trastuzumab. In the preliminary results of 92 patients, ORR was 22.4%, DCR was 57.7%, median PFS was 2.7 months, and median OS was 13.9 months.132
ZW25 is a bispecific antibody that simultaneously binds to two epitopes on HER2: one in extracellular domain 4, containing the trastuzumab binding site, and the other in extracellular domain 2, containing the pertuzumab binding site. Preclinical studies using this agent have demonstrated high levels of anti-tumour activity at a wide range of HER2 expression levels, and that ZW25 is a more effective inhibitor of HER2 signalling than the combination of trastuzumab and pertuzumab because of its improved binding, clustering, and receptor internalisation and downregulation.133,134 In a phase I study in HER2-expressing solid tumours, ZW25 was well tolerated and demonstrated ORR of 67% in BTC, >30% in CRC and gastric or GEJ cancer.135
NJH395 is a bispecific antibody derivative, which is animmune-stimulating antibody conjugate consisting of a monoclonal anti-HER2 antibody conjugated to an immunostimulatory agent.136 A phase I trial designed to determine safety and dose of NJH395 is currently recruiting patients with non-breast HER2-positive advanced-stage cancer (ClinicalTrials.gov identifier: NCT03696771).104 4-1BB (CD137) is a key costimulatory immunoreceptor and promising therapeutic target in cancer. PRS-343, which is a 4-1BB/HER2 bispecific molecule, is designed to facilitate T-cell costimulation by tumor-localized, HER2-dependent 4-1BB clustering and activation.137 In a phase I, dose-escalation study, PRS-343 monotherapy was well-tolerated in all doses and demonstrated objective response in two of 18 evaluable patients (11%) in heavily pre-treated patient with HER2-positive solid tumours.138 Currently, another phase I trial with PRS-343 in combination with atezolizumab in HER2-positive solid tumours, is ongoing (ClinicalTrials.gov identifier: NCT03650348).137
Conclusion
In GI cancers, HER2-targeted therapy is an area of active investigation. Although trastuzumab in advanced gastric or GEJ cancer is the only anti-HER2 agent in GI cancer so far, other novel HER2-targeted agents have emerged and shown promising efficacy in gastric and other GI cancers. Although several issues, including more precise selection of patients to receive HER2-targeted therapies, still need to be addressed for progress in the development of HER2-targeted therapy in GI cancer, novel methods such as screening using ctDNA, basket trials, and an establishment of international diagnostic criteria will likely enable continued advances in HER2-targeted therapy in the future.












