In 1777, Maximilian de Stoll, a Viennese surgeon published the first credited description of gallbladder cancer. Over two centuries later, gallbladder cancer remains a difficult disease with a dismal prognosis for those patients with advanced disease. Historically described as an extrahepatic biliary cancer, gallbladder cancer is now regarded as a distinct disease from other biliary cancers. Despite its relative infrequency, it is the most common cancer of the biliary system. With the advent of laparoscopic cholecystectomy the incidence of early-stage gallbladder cancer has increased, though it is not uncommon for gallbladder cancer to present as advanced disease. As such, it is a challenge for patients and clinicians alike. Here we review the epidemiology, diagnostic techniques, surgical management, adjuvant therapy, and future clinical targets for gallbladder cancer.
Incidence/Epidemiology
Gallbladder cancer is the fifth most common gastrointestinal malignancy in the US and the most common cancer of the biliary system.1 It has an incidence of approximately one to two per 100,000 people, with approximately five to six thousand new cases per year, as estimated by the Surveillance, Epidemiology and End Results (SEER) database. While showing a small decrease in overall incidence over the past 10 years, the incidence in younger populations is growing. Socioeconomic status and access to cholecystectomy have been shown to relate to the incidence gallbladder cancer.2 Its incidence is higher in South American countries, especially Chile, and in populations where cholelithiasis is prevalent.3,4 The incidence of gallbladder cancer is increasing in China and other countries where westernization of diet and increasing obesity are thought to play a role.5,6
Between 10 and 15% of the adult US population is affected by cholelithiasis— the number one risk factor for all biliary cancers—and over 750,000 cholecystectomies are performed annually in the US.7 This has led to an increase in incidentally detected gallbladder cancers, of which approximately 80% are stage I or II.8 It has been estimated that gallbladder cancer found incidentally after cholecystectomy occurs in 0.2–2.9% of cases, representing anywhere from 40 to 70% of all gallbladder cancer diagnoses.9,10
Demographics
A number of demographic variables play a role in gallbladder cancer. Females have a 40 to 60% higher incidence of gallbladder cancer than men.7 Race and ethnicity also play a role, with incidence highest in Caucasian Hispanics and Native Americans, and lowest in non-Hispanic Caucasians and African Americans. The incidence of gallbladder cancer also increases with age, with mean diagnosis in the seventh decade of life.11
Risk Factors
Chronic inflammation of the gallbladder is the most identifiable cause for development of gallbladder cancer.3 While this is most often due to cholelithiasis, any disease process that causes chronic inflammation of the gallbladder will increase the risk for gallbladder cancer. While other extrahepatic biliary cancers are associated with pigmented stones,gallbladder cancer is more common with cholesterol stones.6 Stone size and duration also correlate with gallbladder cancer risk, likely relating to the chronicity of inflammation. Congenital disease processes that lead to inflammation and increase gallbladder cancer risk include primary sclerosing cholangitis, choledochal cysts, and abnormal pancreatobiliary duct junction (APDJ). In APDJ, the pancreatic duct inserts higher on the common bile duct creating a long common channel and leading to increased reflux of pancreatobiliary contents. Comorbid diseases associated with western diet such as obesity and diabetes are also associated with gallbladder cancer.12,13 On the opposite end of the spectrum, nutritional disease states such as rapid weight loss, the metabolic syndrome, and chronic parenteral nutrition cause cholestasis and, as a result, chronic inflammation, possibly increasing cancer risk.14 Chronic inflammation can lead to calcification of the gallbladder wall, termed porcelain gallbladder. Historically, it was thought that these patients had a high likelihood of harboring gallbladder cancer. However, more recent studies have shown the incidence of malignancy to be relatively low at 6–15% among patients with a porcelain gallbladder.15,16
While gallbladder polyps are generally benign, they may be precursors to gallbladder cancer. The risk for gallbladder malignancy in incidental polyps is, however, low—although it appears to increase with size.17 Small polyps (<6 mm) are considered low risk, while those >10 mm or those that are growing in size, particularly in patients >60 years of age, are considered higher risk, and these patients should undergo cholecystectomy.18–21
Drugs and chemicals which cause other biliary cancers are similarly associated with gallbladder cancer, including methyldopa, isoniazid, and carcinogens such as nitrosamines, cigarette smoke, and industrial exposures. Oral contraceptives and hormone replacement therapy have been implicated as risk factors for gallbladder cancer; however, the data are conflicting, and may be due to confounding influences of female sex, obesity, and diet.22,23 Bacterial infection causing chronic inflammation and the production of carcinogenic metabolites is also thought to have a role in the pathogenesis of gallbladder cancer. Salmonella typhi has been well documented in gallbladder cancer but Helicobacter pylori and Escherichia coli have also been implicated, although their relationship is less clear.24,25
Diagnostic Techniques
Ultrasonography
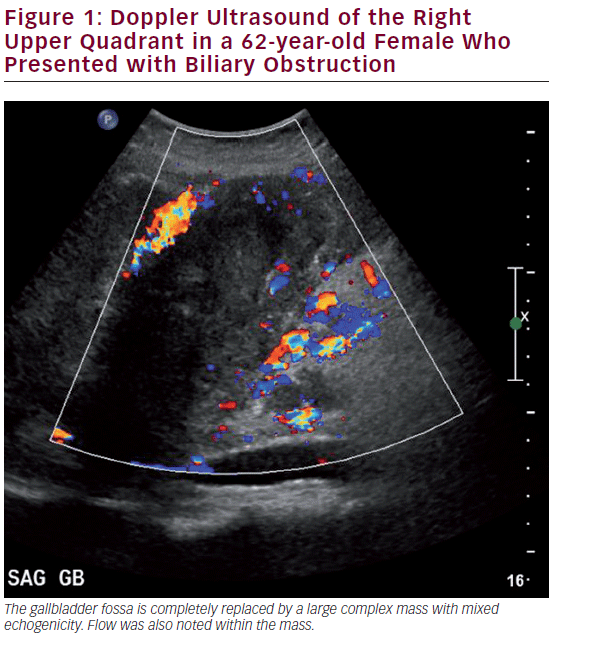
Ultrasound (US) is the most commonly utilized imaging modality to diagnose gallbladder disease (see Figure 1). Gallbladder polyps found during ultrasonography may raise suspicion for malignancy based on other associated patient risk factors/findings. While specificity has been shown to be over 90%, the sensitivity of US in diagnosing gallbladder polyps is poor, correlating with the histopathology of specimens in only 50% of cases.26 US findings such as increased wall thickness, nondependent stones, large polyps (>1 cm), or an underlying mass are highly suspicious and require further investigation. Doppler interrogation is often utilized to assess for the presence of vascular flow within a polyp or mass, and to help in distinguishing these from gallbladder sludge. Endoscopic US (EUS) is more sensitive than transabdominal US, and has the added benefit of determining depth of invasion, extent of local disease, and nodal disease.27 EUS has been shown to accurately diagnose T-stage: pTis 100%, pT1 75.6%, pT2 85.3%, and pT3-4 92.7%.28,29 EUS also adds the possibility of fine needle aspiration (FNA) for tissue diagnosis of the primary as well as lymph nodes, where diagnostic accuracy approaches 100%.30 A recent meta-analysis including 284 patients showed a pooled sensitivity of 84% and a specificity of 100% for diagnosing biliary and gallbladder masses with EUS.31 In general, however, EUS is unnecessary despite its diagnostic accuracy. A suspicious gallbladder mass seen on US or cross-sectional imaging should be surgically removed regardless of the information gained by EUS. For those patients with an advanced nonincidental gallbladder mass where resection is not feasible, EUS may be helpful to obtain a tissue diagnosis to initiate other modalities of therapy.
Cross-sectional Imaging—Computed Tomography and Magnetic Resonance Imaging
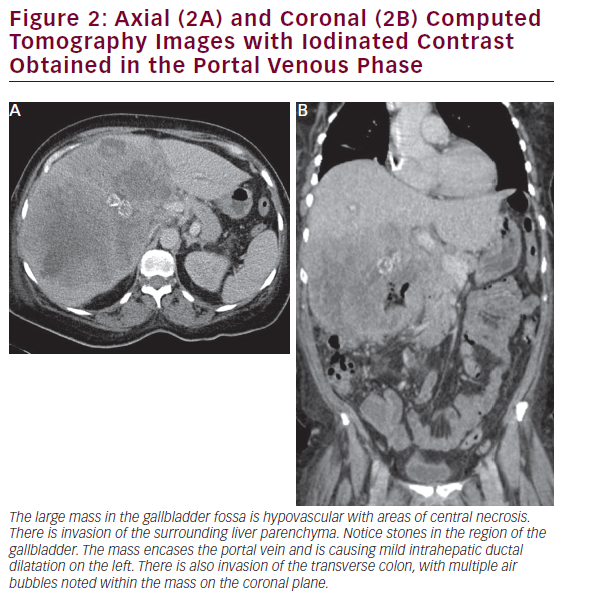
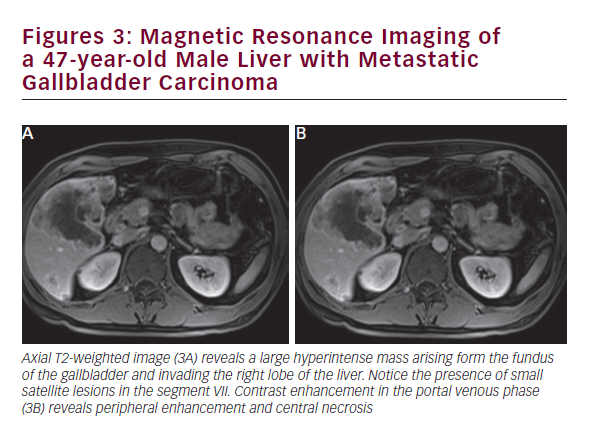
CT is useful in patients with known gallbladder cancer and when combined with multiplanar reconstruction (MPR) can be used to differentiate T-stage with an overall accuracy of more than 80% (see Figure 2).32 CT obtained in the portal venous phase after iodinated contrast administration is also useful to identify concerning features, such as wall thickening, large polyps, and local invasion into the liver, as well as metastatic disease.33 Furthermore, in patients who have undergone resection or those who are deemed unresectable, CT can be employed to monitor for recurrence or response to therapy. MRI is better at identifying smaller lesions and can be useful to help identify the malignant potential of lesions that are otherwise difficult to discern (see Figure 3). Typically, worrisome lesions on MRI display focal wall thickening and an eccentric mass in 76% of cases, and are detectable on T2-weighted sequences at a rate of 78% versus 22% of nonmalignancies.34–36 When combined with MR cholangiography (MRC) and MR angiography (MRA), diagnostic accuracy improves further, with a high sensitivity and specificity of detecting bile duct invasion (100% and 89%), vascular invasion (100% and 87%), hepatic invasion (67% and 89%), and lymph node metastases (56% and 89%).37 MRI obtained with and without gadolinium-based contrast is particular useful in detecting solid enhancing components within the gallbladder. Image subtraction techniques can be utilized to detect subtle enhancement, and confirm the presence of neoplasm.
Positron Emission Tomography
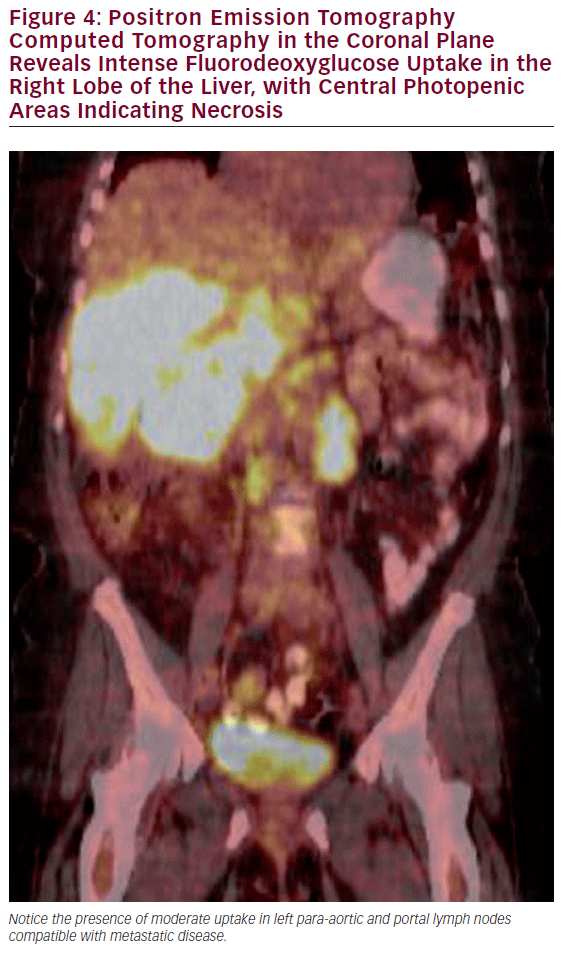
The most common use of PET is with CT for the detection of lymph node involvement and distant disease. The PET CT combination has been shown to have a significantly higher sensitivity than CT alone in detecting metastatic regional or distant disease (see Figure 4).38,39 PET scans can also assist in the evaluation of polypoid lesions >1 cm, as a subset of these will demonstrate fluorodeoxyglucose (FDG) avidity thereby increasing the suspicion of an underlying malignancy. The utility of PET scans to identify residual disease after cholecystectomy in which an incidental gallbladder cancer was subsequently noted is controversial, as postoperative changes often cannot be adequately discerned from malignancy in the gallbladder fossa.
Surgical Management
Some surgeons advocate for routine diagnostic laparoscopy prior to reresection of incidental tumors that are greater than T1 after cholecystectomy. Several studies have shown a significant number of patients are unresectable on re-exploration, and diagnostic laparoscopy avoids the morbidity of a large operation.40–42 As such, diagnostic laparoscopy should be considered in patients—especially patients with advanced T stage and an elevated CA19-9.
The extent of parenchymal resection should be dictated by the T stage of the gallbladder cancer and the ability to obtain an R0 resection. For patients with T1a tumors, a simple cholecystectomy with negative margins is sufficient and these patients do not need further surgical intervention. The rate of lymph node metastases has been reported to be less than 2.5%, so lymphadenectomy is generally not warranted.43,44 Patients with T1a disease who undergo simple cholecystectomy alone can be expected to have a disease-free survival between 90 and 100%. By contrast, the management of T1b tumors is more controversial. For T1b lesions, a simple cholecystectomy may be sufficient as long as negative margins are achieved.45 However, several studies have shown a survival benefit with more extensive resection.46 Hari et al. reported an improved 5-year survival of 50% to 79% in patients undergoing radical cholecystectomy for T1b lesions.43 While these findings may reflect stage-migration with more adequate surgical staging, nonetheless, it is important to note that 15% of patients with T1b disease had lymph node metastasis.43 Several other studies have similarly suggested that repeat surgery with a liver resection confers a recurrence-free survival advantage in patients with T1b lesions.8,47–52
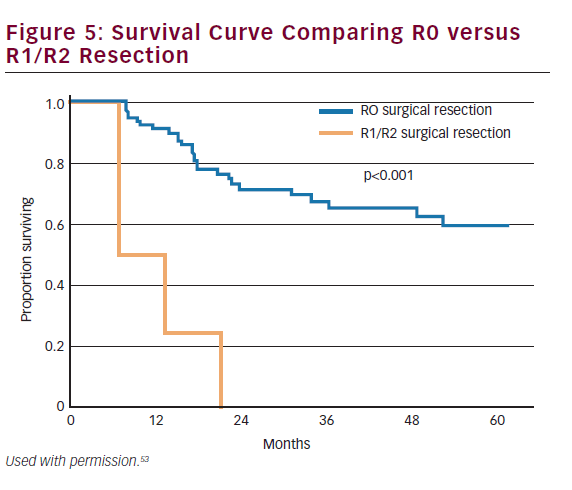
The majority of gallbladder cancers are T2 or T3 at the time of diagnosis. These patients will either present incidentally or with a nonincidental mass. Patients with T2 or T3 tumors require repeat hepatic resection with goals of achieving an R0 resection. Surgical margin status is one of the most important predictive measures of long-term outcome (see Figure 5).53,54 While anatomic hepatic resection must be considered when necessitated by the extent of the mass or concomitant vascular involvement, routine extended hepatic resection has not been shown to increase survival over nonanatomic resection and may be associated with increased morbidity and mortality.55 Therefore, for most patients, repeat hepatic resection will consist of a segment IVb and V resection. For selected patients, extended hepatectomy may be necessary to achieve negative margins and has been shown to be safe.46 Among patients scheduled to undergo an extended hepatic resection, but who have an inadequate future liver remnant (i.e. less than 20–30%), preoperative portal vein embolization can be employed to induce hypertrophy of the future liver remnant prior to surgery.56 Among patients with T2 or T3 cancers, ‘radical’ repeat surgery has been shown to improve survival compared with simple cholecystectomy.57
While some studies have suggested that lymphadenectomy, in and of itself, has a survival benefit, these findings are probably related to staging bias.58–61 Lymphadenectomy, however, remains an important component of surgery for gallbladder cancer. Adequate lymph node assessment contributes to adequate staging and may decrease the risk for local recurrence. The extent of lymphadenectomy undertaken at the time of surgery varies. Surgeons in the US generally perform a regional lymphadenectomy that includes the perihilar nodes (cystic duct, common bile duct, hepatic artery, and portal vein). In Asian countries, surgeons tend to perform a lymphadenectomy that includes these basins as well as the celiac axis, superior mesenteric artery, and pancreaticoduodenal and aortocaval regions in select cases.62 While some data have suggested that a more extensive lymphadenectomy may improve outcomes,63 nodal disease in certain basins such as the para-aortic region portends aggressive tumor biology and a particularly poor outcome.64 In addition to the location, the number of lymph nodes retrieved is also important for both locoregional disease control as well as staging. In general, retrieval of at least three lymph nodes is recommended by the AJCC-UICC. Despite this recommendation, studies have shown that a large percentage of patients often do not undergo an adequate lymphadenectomy.62,65
As with most cancers, lymph node involvement is a poor prognostic sign regardless of T stage.57,66 The AJCC (7th edition) separates lymph node metastases into local (N1: cystic duct, common bile duct, hepatic artery, and/or portal vein) and distant (N2: periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes). Patients with N2 disease are categorized as stage IVb, and have a survival of less than 2% at 5 years. The number of positive lymph nodes (0,1 to 3 or >3) has also been shown to have prognostic capability as well.62,67
Another consideration when operating on patients with gallbladder cancer is how to manage the common bile duct. Some surgeons suggest routine resection of the common bile duct at the time of hepatic resection and portal lymphadenectomy,68–71 while others have advocated only selective resection of the common bile duct.53,72 Several studies have noted that the median number of lymph nodes harvested at the time of lymphadenectomy was the same regardless of whether the common bile duct was or was not resected concomitantly with the lymph node dissection.53,72,73 In addition, resection of the common bile duct plus lymphadenectomy does not seem to be associated with an improvement in survival.53,72,73 As such, a routine common bile duct resection is probably not warranted when the cystic duct margin is free of disease. However, a positive cystic duct margin has been strongly associated with residual disease in the common bile duct and therefore a bile duct resection with hepaticojejunostomy is indicated in this setting.53
Even in patients with a preoperative diagnosis of early-stage gallbladder cancer, the incidence of spillage of gallbladder contents may be as high as 25–36%.74 The potential risk for seeding the peritoneal cavity has led many to recommend open operation for all patients with a preoperative diagnosis of gallbladder cancer. Laparoscopic resection has been associated with a higher recurrence rate, a higher port-metastasis rate, and worse survival rates compared with open cholecystectomy.74 However, as laparoscopic techniques for hepatic resection have improved, recent studies have suggested laparoscopy to be a safe alternative in selected patients. Laparoscopic re-resection after incidental gallbladder cancer diagnosis has been described in selected patients without affecting morbidity or mortality. While growing data suggest that a laparoscopic approach may be safe and effective, and permit an adequate hepatic resection and lymphadenectomy,75,76 this approach should only be used in selected patients by surgeons highly trained in laparoscopic techniques.61,77
Port-site resection has not been shown to improve overall survival or recurrence-free survival. Rather, port-site metastasis serves as an indicator of underlying disseminated disease, even occurring in up to 19% of patients who underwent port-site resection.78 Routine port-site resection is therefore not necessary.79

Other Surgical Considerations
Preoperative Jaundice
While jaundice is a poor prognostic factor in patients with gallbladder cancer, the presence of jaundice does not necessarily preclude operative intervention as long as negative margins may be achieved.80 Feng et al. advocate radical resection when possible in those with jaundice as median survival in these patients approaches those without jaundice.81 However, other investigators have cautioned that preoperative jaundice portends a poor prognosis and have described preoperative jaundice as a strong relative contraindication to resection.82 If jaundiced patients are in fact deemed to be unresectable, these patients should generally undergo biliary decompression using either percutaneous biliary drain placement or endoscopic stenting. In turn, these patients should be referred for consideration of chemotherapy/ chemoradiation if commensurate with the patient’s performance status.83
Intraoperative Suspicion
While gallbladder cancer is commonly discovered only after routine laparoscopic cholecystectomy on final pathology, intraoperative diagnosis is also possible. Intraoperative predictors of incidental gallbladder cancer include age over 65, dilated intra- or extrahepatic biliary system, and gallbladder wall thickening.84 Those patients in whom suspicion is raised intraoperatively should almost always be converted to an open procedure followed by definitive management, including intraoperative US, hepatic resection, and lymphadenectomy. At centers where definitive management is not feasible, the procedure should be aborted and the patient sent to a facility with expertise in biliary cancers. Data have suggested that delaying operative management and referring the patient to an experienced center is the best therapeutic approach.85
Pathology and Staging
The majority of gallbladder cancers (80–90%) are adenocarcinomas, arising from the mucosa. Other types include squamous, adenosquamous and small cell cancers. Their morphology ranges from papillary, which has the most favorable prognosis, to infiltrative, which has the worst prognosis. The lack of a mucosal muscularis layer allows the early spread of these cancers, both loco-regionally and systemically. The most recent AJCC staging system (see Tables 1 and 2) reclassified nodal disease as either N1 (adjacent to the porta hepatis) and N2 (distant nodal disease) and included tumors arising from the cystic duct.86
Prognosis
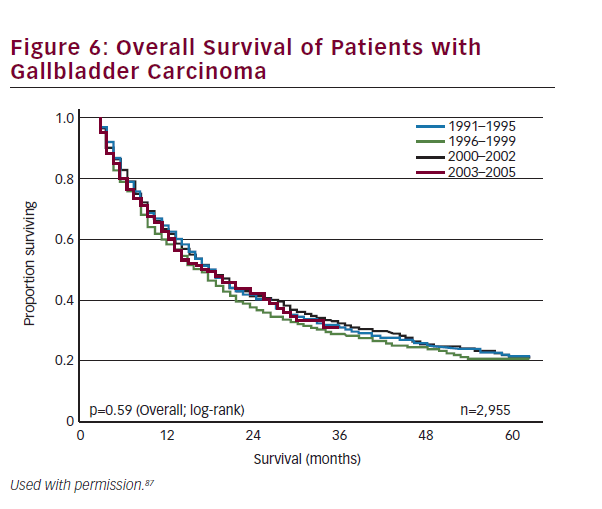
Historically, overall prognosis has been poor due to the advanced stage at presentation. Recent trends in earlier diagnosis through laparoscopic cholecystectomy and aggressive surgical management have improved survival across all stages of the disease. Survival is strongly correlated to stage and resectability. Overall 1-, 3-, and 5-year survival for all patients with gallbladder cancer has been reported at 56%, 30%, and 21%, respectively (see Figure 6).87 Patients who have early-stage disease and those patients who have an R0 resection have the best prognosis. The 5-year survival of patients with T1 and T2 disease has been reported to range from 79–100% and 42–90%, respectively.43,53,68,85,88,89 By contrast, patients with T3 lesions have a worse prognosis with most series reporting 5-year survival ranging from 33 to 45%.68,85,88,90 As noted, metastatic nodal disease also portends a poor prognosis with 5-year survival among patients with lymph node metastasis being about 20–50%.53,91–94 Other studies have noted that margin status is similarly associated with survival.53,95 Despite advancements in the multimodality therapy of gallbladder cancer, patients who present with nonincidental gallbladder cancers have a particularly poor prognosis with 5-year survival rates ranging between 0 and 20%.8,53,54,96,97
Chemotherapy and Radiation Therapy
The role of adjuvant chemotherapy in the treatment of gallbladder cancer is difficult to define. More often than not, gallbladder cancer is grouped with other cancers of the biliary tract, and therefore data on adjuvant therapy are often not specific. In addition, most reports relating to the efficacy of adjuvant therapy are underpowered retrospective studies. Although there have been no prospective randomized trials evaluating adjuvant therapy in only patients with gallbladder cancer, a few studies have exhibited benefit for these patients in subgroup analysis. A prospective study by Takada et. al involving a cohort of patients with pancreaticobiliary cancer showed a significant improvement of 5-year survival with mitomycin-C and 5-fluorouracil versus surgery alone (26.0% versus 14.4%) in a subgroup analysis of 140 patients with gallbladder cancer.98 Other prospective studies have noted a survival benefit in patients with gallbladder cancer receiving adjuvant chemotherapy.99,100 Current NCCN guidelines recommend adjuvant therapy with flouropyrimidine-based chemotherapy in combination with radiation therapy for gallbladder cancer stage II or higher.101 The role for adjuvant chemotherapy is supported by several retrospective studies.99,102–104 Several other retrospective studies have reported that adjuvant radiation with concurrent chemotherapy shows significant improvement in both disease-free and overall survival.105–107 Few studies relate to the role of neoadjuvant therapy, however, these data have noted an improvement in survival.108
For patients with advanced inoperable gallbladder cancer, systemic and locoregional therapeutic options should be considered. Patients with advanced disease should be treated with systemic chemotherapy, typically with a platin-based regimen. Alternative therapies have been proposed, including mitomycin C/5-flourouracil, which have been reported to have some activity.109 S-1, which is not currently available in the US, has also shown promise in those with stage IV cancers of the biliary tract, including gallbladder, with a 30.4% response rate.110–112 In addition, for those patients with no evidence of metastatic disease, radiation therapy may have a benefit in terms of improving local control. For patients with stage IV cancers, the overwhelming majority are not surgical candidates. While rare, there are reports of metastectomy for gallbladder cancer that have suggested a possible survival benefit.113–116 These data, however, need to be interpreted cautiously and it needs to emphasized that the overwhelming majority of patients with stage IV disease should not undergo resection, and should instead be referred for systemic therapy.117
Future Targets
Definitive targeted therapy for gallbladder cancer remains elusive. Vascular endothelial growth factor (VEGF)-alpha expression on resected specimens has correlated with metastatic disease, and worse overall prognosis.118 Still, response to targeted therapy has not shown much promise in treatment of gallbladder cancer.119 Several genes have been shown to play a role in gallbladder cancer. Both p53 Upregulated Modulator of Apoptosis (PUMA) and C-myb nuclear oncogene have been identified in gallbladder cancer. The extent of expression has been correlated to the biologic aggressiveness of the tumor as well as overall survival.120 Other candidate genes identified in relation to gallbladder cancer include p53, COX2, XPC, RASSF1A, adenosine triphosphatebinding cassette transporter ABCG8, and membrane-bound enzyme ADAM-17. These genes are being studied and hopefully more effective targeted therapy can be identified in the future.121
Conclusion
Carcinoma of the gallbladder is often an aggressive disease with a poor prognosis for patients with advanced disease. Patients who have earlystage disease and who undergo an R0 resection have a significantly better prognosis. Resection to negative margins with lymphadenectomy offers the only hope for long-term survival. Adjuvant chemotherapy and chemoradiation should be strongly considered for patients with stage II disease or greater, especially in the setting of an R1 margin. Given that response rates to chemotherapy remain poor, future research will need to identify more effective therapeutic options in order to improve the outcome of patients with gallbladder cancer.













