Oesophageal cancer (OC) is the seventh most common malignancy and the sixth leading cause of death from cancer worldwide,1 with approximately 604,000 new cases in 2020.2 OC has two main histological subtypes: oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC). Worldwide, OSCC accounts for approximately 90% of all cases of oesophageal carcinoma and OAC for approximately 10%.3 OSCC is the most common subtype in East Asia and East Africa, and rates of OAC are higher in Western countries compared with rates of OSCC.4–6 OC is sometimes asymptomatic in the early stages, and is often diagnosed at an advanced stage;7 therefore, the prognosis of patients with OC remains poor. Systemic treatment in the advanced metastatic setting achieves a median overall survival (OS) of 10–12 months.8,9 Doublet chemotherapy consisting of cisplatin and fluoropyrimidine is recognized as standard first-line chemotherapy for metastatic or recurrent OC in the palliative setting,3,10–12 and monotherapy with a taxane or irinotecan is used as later-line chemotherapy.13–15
Immune checkpoint inhibitors (ICIs) have recently become a standard treatment for several malignancies. Nivolumab, a human monoclonal antibody that targets programmed cell death protein-1 (PD-1), was established as a second-line treatment for patients with metastatic or recurrent OSCC in the ATTRACTION-3 trial.16 Pembrolizumab, another PD-1 antibody, has been approved as second-line chemotherapy for only advanced or metastatic OSCC with a programmed cell death protein-1 ligand (PD-L1) combined positive score of ≥10, based on the results of the phase 2 KEYNOTE-180 trial and the phase 3 KEYNOTE-181 trial.17,18 Later, the KEYNOTE-590 trial also showed a significant survival benefit in the first–line setting.19 However, 27% of the subjects in that trial had OAC and not only OSCC. Furthermore, in the CheckMate 648 trial, OS was significantly longer in patients with OSCC who received nivolumab in addition to cisplatin/5-fluorouracil or ipilimumab, than in those who received chemotherapy alone.20 This review discusses the current status and future perspectives of nivolumab combination therapy for patients with advanced OSCC.
Nivolumab
Nivolumab is an engineered human immunoglobulin G4 monoclonal antibody that binds to PD-1 and blocks the interaction of PD-1 with its ligands PD-L1 and PD-L2, thereby alleviating suppression of the anti-tumour immune response via the PD-1 pathway.21 PD-1 and PD-L1 play an important role in modulating the immune response to cancer cells. PD-1 is an immunosuppressive receptor that is highly expressed on immune cells, including activated T cells, B cells and natural killer cells.22 When PD-1 binds to PD-L1 on cancer cells, these cancer cells avoid the anti-tumour immune response. Therefore, an anti-PD-1 antibody prompts anti-tumour activity by inhibiting the interaction of PD-1 with its ligands PD-L1 and PD-L2 on activated lymphocytes.23 PD-L1 expression is enriched in OSCC,24 and its overexpression is detected in 18.4–82.8% of patients with the disease.25 Nivolumab is approved for the treatment of various cancers, including melanoma, non-small-cell and small–cell lung cancer, gastric cancer, renal cell carcinoma, Hodgkin lymphoma, squamous cell carcinoma of the head and neck, urothelial carcinoma, hepatocellular carcinoma and microsatellite instability-high/mismatch–repair deficient colorectal cancer.26–35
Nivolumab as second- or later-line treatment
Nivolumab has been developed for the treatment of many cancers, including OC. Two trials have examined the efficacy and safety of nivolumab in OC after second-line treatment.16,36 Table 1 summarizes the recent clinical trials that have investigated the clinical benefit of nivolumab.16,20,36–39
Table 1: Results of clinical trials of nivolumab in patients with metastatic or recurrent oesophageal cancer
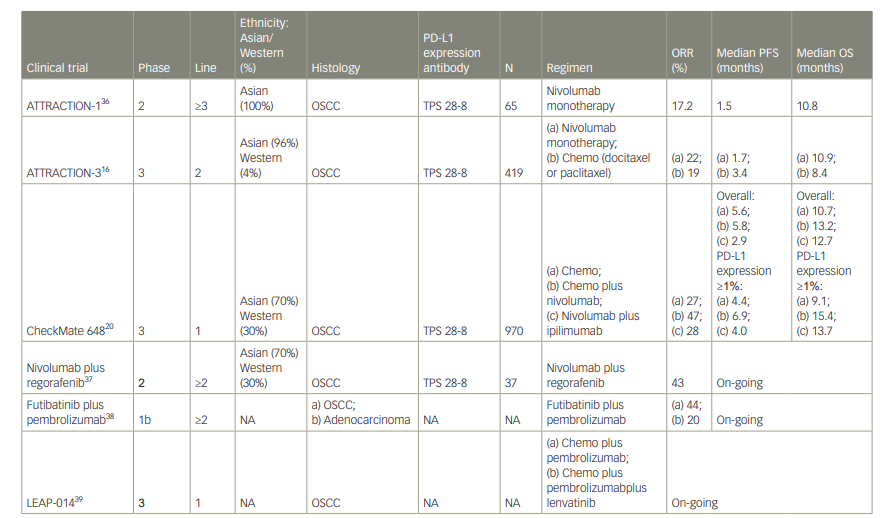
chemo = chemotherapy; NA = not applicable; ORR = objective response rate; OS = overall survival; OSCC = oesophageal squamous cell carcinoma; PD-L1 = programmed cell death protein-1 ligand; PFS = progression-free survival; TPS = tumour proportion score.
The ATTRACTION-1 trial
This study was a multicentre, open-label, single-arm, phase 2 clinical trial conducted in Japan to evaluate the clinical activity and safety of nivolumab monotherapy (3 mg/kg, every 2 weeks) in patients with advanced or metastatic OC after failure of fluoropyrimidine-based, platinum-based and taxane-based chemotherapy, regardless of PD-L1 expression status.36 The primary endpoint was the centrally assessed objective response rate and the secondary endpoints included OS, progression-free survival (PFS), time to response and duration of response. Sixty-five patients were enrolled in the trial; 64 were evaluable for efficacy and all patients were evaluable for safety. By central adjudication, a response was observed in 11 of the 64 patients, giving a response rate of 17.2% (95% confidence interval [CI] 9.9–28.2). The median duration of response was 11.2 months (95% CI 3.02–not reached [NR]), and the median time to response was 1.45 months (95% CI 1.4–3.0). Median OS was 10.8 months (95% CI 7.4–13.3) and median PFS was 1.5 months (95% CI 1.4–2.8).
The most common grade 3 or 4 events were lung infection (8%), decreased appetite (3%), increased blood creatinine phosphokinase (3%), dehydration (3%), dyspnoea (2%) and hyponatraemia (2%). There were no treatment-related deaths.
Overall, these data show that nivolumab monotherapy has promising efficacy, with manageable adverse events in patients with advanced OC.
The ATTRACTION-3 trial
Based on the results of ATTRACTION-1, the multicentre, global, phase 3 ATTRACTION-3 trial was conducted. In this trial, 419 patients with advanced OSCC were randomized to receive nivolumab (240 mg every 2 weeks until disease progression or unacceptable toxicity) or chemotherapy at the investigator’s discretion (paclitaxel 100 mg/m2 once per week for 6 weeks then 1 week off; or docetaxel 75 mg/m2 every 3 weeks) as second-line treatment after failure of fluoropyrimidine-based chemotherapy.16 The patients were included regardless of PD-1 status and were assigned 1:1 to nivolumab or chemotherapy (paclitaxel or docetaxel). The primary endpoint was OS.
Median OS was 10.9 months (95% CI 9.2–13.3) in the nivolumab group versus 8.4 months (95% CI 7.2–9.9) in the chemotherapy group, and was significantly longer in the nivolumab group (hazard ratio [HR] 0.77, 95% CI 0.62–0.96; p=0.019) than in the chemotherapy group. The response rate was similar in the nivolumab group and the chemotherapy group (19% [95% CI 14–26] versus 22% [95% CI 15–29], respectively). However, the duration of response was longer in the nivolumab group than in the chemotherapy group (6.9 months versus 3.9 months, respectively). PFS was 1.7 months (95% CI 1.5–2.7) for nivolumab and 3.4 months (95% CI 3.0–4.2) for chemotherapy (HR 1.08, 95% Cl 0.87–1.34).
In a subgroup analysis, the nivolumab group showed better outcomes than the chemotherapy group for all risk factors. Analysis of PD-L1 expression showed that median OS in the nivolumab group was longer compared with the chemotherapy group, for both a tumour with PD-L1 <1% (10.9 months [95% CI 8.4–13.9] versus 9.3 months [95% CI 7.2–12.0], respectively; HR 0.84 [95% CI 0.62–1.14]) and a tumour with PD-L1 ≥1% (10.9 months [95% CI 8.0–14.2] versus 8.1 months [95% CI 6.0–9.9], respectively; HR 0.69 [95% CI 0.51–0.94]).
The most common treatment-related adverse events were skin rash (11%), diarrhoea (11%) and anorexia (8%) in the nivolumab group, and alopecia (47%), neutropenia (37%) and leukopenia (35%) in the standard chemotherapy group. The incidence of treatment-related serious adverse events was lower in the nivolumab group than in the standard chemotherapy group. The treatment-related serious adverse event rate was 16% in the nivolumab group and 23% in the standard chemotherapy group.
Based on the results of ATTRACTION-3, the United States Food and Drug Administration approved nivolumab monotherapy on 10 June 2020 as a treatment for advanced OSCC that is refractory to primary treatment with platinum-based chemotherapy and fluoropyrimidine, regardless of PD-L1 expression status.40
Nivolumab as first-line treatment
CheckMate 648 was a randomized phase 3 trial that evaluated the efficacy of nivolumab in combination with chemotherapy, and nivolumab combined with ipilimumab (an anti-cytotoxic T-lymphocyte-associated protein 4 antibody) as first-line therapy.20 The study included only patients with untreated advanced OSCC. The primary endpoints were PFS and OS in patients with PD-L1 positivity (tumour proportion score [TPS] ≥1). Secondary endpoints were PFS and OS in all patients and the objective response rate in patients with PD-L1 positivity and in all patients. In this trial, 970 patients were randomized 1:1:1 to receive nivolumab plus chemotherapy, nivolumab plus ipilimumab, or chemotherapy alone; 70% were Asian, and approximately 50% were PD-L1 positive. OS was 15.4 months for patients with PD-L1 positivity and advanced OSCC in the nivolumab plus chemotherapy group versus 9.1 months for their counterparts in the chemotherapy group (HR 0.54 [95% CI 0.37–0.80]; p<0.0001), demonstrating the superiority of the nivolumab combination. For patients with PD-L1 positivity, the superiority of nivolumab plus ipilimumab was also demonstrated in terms of OS (13.7 months versus 9.1 months in the chemotherapy group; HR 0.64 [95% CI 0.46–0.90]; p=0.001). Furthermore, OS in all patients was 13.2 months in the nivolumab plus chemotherapy group versus 10.7 months in the chemotherapy group (HR 0.74 [95% CI 0.58–0.96]; p=0.002) and 12.7 months in the nivolumab plus ipilimumab group versus 10.7 months in the chemotherapy group (HR 0.78 [95% CI 0.62–0.98]; p=0.01). Median PFS in patients with PD-L1 positivity was 6.9 months in the nivolumab plus chemotherapy group versus 4.4 months in the chemotherapy group (HR 0.65 [95% CI 0.46–0.92]; p=0.002). However, median PFS in PD-L1-positive patients was 4.0 months in the nivolumab plus ipilimumab group versus 4.4 months in the chemotherapy group (HR 1.02 [95% CI 0.73–1.43]; p=0.90); the difference was not statistically significant.
The treatment-related, all-grade adverse event rate was 96% in the nivolumab plus chemotherapy group, 90% in the chemotherapy group and 80% in the nivolumab plus ipilimumab group. The respective rates of grade 3 or higher events in the nivolumab plus chemotherapy group, the chemotherapy group and the nivolumab plus ipilimumab group were 47%, 36% and 32%. Immune-related adverse events tended to be more common in the nivolumab plus ipilimumab group and included rash (17.1%), pruritus (13.4%) and hypothyroidism (13.4%), but less than 6% of these patients had grade 3 or higher adverse events, which is comparable to data previously reported for other types of cancer.41,42
A Japanese subgroup analysis demonstrated the superiority of nivolumab in terms of OS in patients with OSCC TPS ≥1 (17.3 months in the nivolumab plus chemotherapy group versus 9.0 months in the chemotherapy group; HR 0.53 [95% CI 0.35–0.82]).43 The superiority of nivolumab plus ipilimumab was also demonstrated (OS 20.2 months versus 9.0 months in the chemotherapy group; HR 0.46 [95% CI 0.30–0.71]). Furthermore, OS in all patients was 15.5 months in the nivolumab plus chemotherapy group versus 11.0 months in the chemotherapy group (HR 0.74 [95% CI 0.54–0.99]) and 17.6 months in the nivolumab plus ipilimumab group versus 11.0 months in the chemotherapy group (HR 0.68 [95% CI 0.51–0.92]). The median PFS in patients with OSCC TPS ≥1 was 7.0 months in the nivolumab plus chemotherapy group versus 4.2 months in the chemotherapy group (HR 0.56 [95% CI 0.36–0.89]), but was 5.4 months in the nivolumab plus ipilimumab group versus 4.2 months in the chemotherapy group (HR 0.84 [95% CI 0.54–1.32]), which was not significantly different. No new safety issues were identified in the Japanese subgroup analysis.
Based on the results of CheckMate 648, nivolumab plus chemotherapy and nivolumab plus ipilimumab combination therapy have become established first-line treatments for patients with untreated advanced OSCC (Figure 1). However, there are some important clinical questions about ICI-containing treatments.
Figure 1: Treatment flow for patients with stage 4b and recurrent oesophageal squamous cell carcinoma
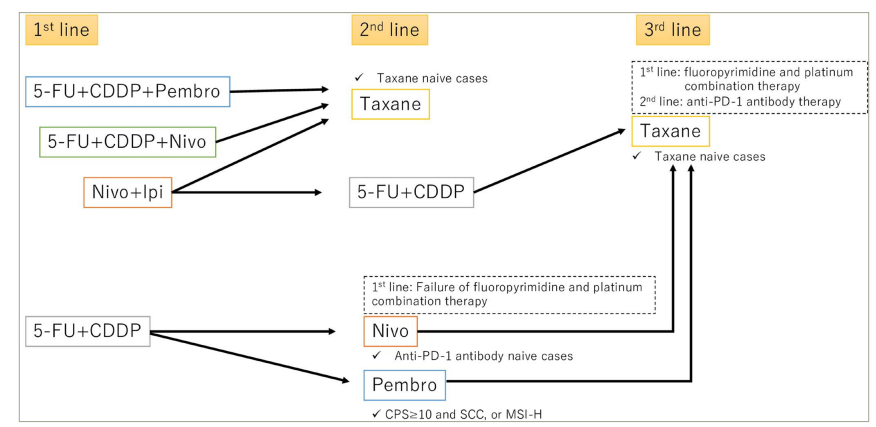
5-FU = 5–fluorouracil; CDDP = cisplatin; CPS = combined positive score; Ipi = ipilimumab; MSI-H = microsatellite instability-high; nivo = nivolumab; pembro = pembrolizumab; PD-1 = programmed cell death protein-1; SCC = squamous cell carcinoma.
First, which regimen is the optimal first-line treatment for patients with untreated advanced OSCC? Although pembrolizumab plus doublet chemotherapy, nivolumab plus doublet chemotherapy, and nivolumab plus ipilimumab combination therapy are available, there are no data directly comparing the efficacy of these three regimens. The expanded analysis of CheckMate 648 identified several clinical factors, including a high tumour burden and liver metastasis, that seemed to be related to the delayed benefits of nivolumab plus ipilimumab combination therapy in patients with untreated advanced OSCC.44 Furthermore, in the population with PD-L1 TPS <1%, the HR for OS was 0.96 for nivolumab plus ipilimumab combination therapy compared with 5-fluorouracil plus cisplatin therapy.44 Therefore, nivolumab plus ipilimumab combination therapy might be beneficial for patients with untreated advanced OSCC, a low tumour burden, PD-L1 TPS ≥1% and no liver metastasis. On the other hand, if disease progression occurs within 6 months after post–operative nivolumab therapy, the OC should be considered nivolumab resistant. Cytotoxic drugs should be considered as palliative chemotherapy. Furthermore, there is no direct comparison on clinical outcomes between nivolumab and pembrolizumab. There is a difference in that nivolumab is given every 2 weeks and pembrolizumab every 3 weeks in clinical trials, but there are no settled opinions for selecting between these two ICIs.
Second, what biomarkers would be useful for choosing between these ICI-containing regimens for patients with untreated advanced OSCC? Several clinical trials, including ATTRACTION-3, KEYNOTE-590 and CheckMate 648, have investigated the relationship between PD-L1 expression (TPS/combined positive score) and efficacy, but their results have not been consistent. The balance of PD-1 expression between effector T cells and regulatory T cells was identified to be a promising biomarker for the efficacy of ICIs in gastric cancers and lung cancers.45 At the 2022 European Society for Medical Oncology congress, Japanese researchers reported the clinical value of a balance of PD-1 expression between effector T cells and regulatory T cells for retrospectively predicting the efficacy of nivolumab monotherapy in patients with previously treated advanced OSCC.46 In that study, the ratio of PD-1 expression in regulatory-T cells/effector T cells was significantly higher in non-responders than in responders (p=0.036). Median PFS was significantly longer in the low-ratio group than in the high-ratio group (3.2 months versus 1.8 months; HR 0.56 [95% CI 0.34–0.92]; p=0.02). Furthermore, median OS tended to be longer in the low-ratio group than in the high-ratio group (NR versus 10.2 months; HR 0.64 [95% CI 0.31–1.30]; p=0.21).
The PD-1 expression ratio in regulatory T cells/effector T cells is biologically plausible, and a biomarker analysis by Mikuni et al.46 agreed with theoretical results in patients with treated advanced OSCC. This biomarker is expected to be useful in patients with untreated advanced OSCC, and might identify the appropriate population for nivolumab plus ipilimumab combination therapy. Further prospective biomarker studies are needed to identify the optimal treatment strategy for patients with untreated advanced OSCC.
Future perspectives
Recently, some ICI investigational treatments have been developed. A phase 1 trial evaluating fusibatinib plus pembrolizumab combination therapy showed an objective response rate of 44% in the ICI-naïve advanced OSCC cohort and 20% in the ICI-refractory advanced OSCC cohort.38
In addition to the mainstream combination of ICIs and chemotherapy for patients with OSCC, the combination of ICIs with molecular-targeted agents is also being assessed. A multicentre, phase 2 study evaluating regorafenib plus nivolumab combination therapy showed an objective response rate of 43% in the treated advanced OSCC cohort.37 ICI combination trials are on–going, namely: the LEAP-014 study, an open-label, randomized phase 3 trial of lenvatinib and pembrolizumab plus chemotherapy as first-line therapy in OSCC (ClinicalTrials.gov identifier: NCT04949256),39 and the KEYMAKER-06 phase 1/2 trial evaluating the safety and efficacy of cytotoxic chemotherapy and/or several second-line treatments consisting of pembrolizumab, anti-lymphocyte activation gene 3 antibody, anti-immunoglobulin-like transcript 4 antibody and lenvatinib (ClinicalTrials.gov identifier: NCT05342636).47 Therefore, these promising investigational treatments are expected to improve clinical outcomes of patients with advanced OSCC.
Conclusions
After nivolumab monotherapy demonstrated efficacy as a second- or later-line treatment, such as in the ATTRACTION-1 and ATTRACTION-3 trials, nivolumab combination therapies have been established as the first-line treatment for patients with untreated advanced OSCC patients based on the CheckMate 648 trial. However, pembrolizumab plus therapy is also an established first-line treatment based on the KEYNOTE-590 trial. Further studies are needed to help choose between three standard treatments, and to identify useful biomarkers.












