Esophageal cancer is the seventh most common malignancy and the sixth leading cause of death from cancer worldwide.1 The main histological subtypes are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. ESCC is the most common subtype, and the frequency of this disease is highest in Eastern Asia and Eastern Africa.2 In Western countries, however, the incidence of esophageal adenocarcinoma is higher than that of ESCC.2–5 The key risk factors associated with ESCC are smoking and alcohol consumption, while the key risk factors of esophageal adenocarcinoma are gastroesophageal reflux disease, obesity, and Barrett esophagus.5,6
Patients with early-stage esophageal cancer are often asymptomatic, but the majority of patients are diagnosed with advanced disease and have a poor prognosis.5,6 In addition, there are few treatment options for metastatic or recurrent patients with esophageal cancer. Doublet chemotherapy, consisting of fluoropyrimidine and platinum, is recognized as the first-line chemotherapy,7,8 and taxane or irinotecan monotherapy was used as salvage-line chemotherapy before the development of immune checkpoint inhibitors.9–11 No targeted agents have been developed for esophageal cancer, and this disease has been understudied in clinical trials over the past several decades.
Introduction to nivolumab
Immune checkpoint inhibitors, such as anti-programmed cell death protein 1 (PD-1) antibody, were first used in esophageal cancer to treat metastatic or recurrent disease. PD-1 is an immune checkpoint receptor that is mainly expressed on cytotoxic T cells. The two ligands for PD-1, programmed death ligand 1 (PD-L1) and PD-L2, are expressed on the surface of cancer cells and immune cells in the tumor microenvironment. In the tumor microenvironment, the binding of these ligands to PD-1 suppresses the activation of cytotoxic T cells, which lose their ability to infiltrate the tumor and kill cancerous cells. The fully-humanized immunoglobulin G (IgG)4 monoclonal PD-1 antibody, nivolumab, binds to PD-1 on cytotoxic T cells and inhibits its interaction with the tumor cell PD-L1 and PD-L2 ligands, thereby blocking the transmission of immunosuppressive signals and maintaining T-cell activation, resulting in an anti-tumor effect.12-15 Nivolumab is effective in a variety of cancer types—the US Food and Drug Administration (FDA) have approved nivolumab for the treatment of advanced melanoma, advanced non-small cell lung cancer, advanced small cell lung cancer, classical Hodgkin lymphoma, advanced renal cell carcinoma, urothelial carcinoma, squamous cell carcinoma of the head and neck, microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer, and hepatocellular carcinoma.16–26
In some clinical trials of immune checkpoint inhibitors, PD-L1 is reported as the candidate of predictive biomarker. Previous reports revealed that PD-L1 overexpression was found in approximately 15–83% of patients with ESCC, and high PD-L1 expression was associated with poor overall survival (OS).27,28 To evaluate PD-L1 expression, tumor proportion score (TPS) or combined positive score (CPS) are often used in clinical trials. TPS is defined as the number of PD-L1 positive tumor cells divided by the total number of tumor cells, multiplied by 100. On the other hand, CPS is defined as the number of PD-L1 positive tumor cells, macrophages, and lymphocytes divided by the total number of tumor cells, multiplied by 100.
The PD-L1 IHC 28-8 pharmDx assay (Dako, an Agilent Technologies, Inc. company, Santa Clara, CA, USA) is mainly used for evaluating TPS in clinical trials of nivolumab, while PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA, USA) is mainly used for evaluating CPS is mainly used in clinical trials of pembrolizumab. PD-L1 expression (TPS) evaluated by 28-8 and 22C3 has shown concordance for patients with lung cancer in the BluePrint project;29 however, there has been no evidence of concordance between TPS and CPS, 28-8 and 22C3 for patients with esophageal cancer.
Nivolumab monotherapy as second-line treatment for patients with advanced esophageal squamous cell carcinoma
Nivolumab has been developed for the treatment of esophageal cancer, as well as other cancers. The phase II ATTRACTION-1 trial was a single-arm study that evaluated the efficacy of nivolumab monotherapy (3 mg/kg every 2 weeks) in patients with metastatic or recurrent ESCC, esophageal adenocarcinoma, or adenosquamous-cell carcinoma of the esophagus who were refractory or intolerant to fluoropyrimidine-based, platinum-based, and taxane-based chemotherapy. Although, ESCC, adeno, and adenosquamous cell patients were eligible to take part in this trial, all 65 patients enrolled in this trial had ESCC as a result (Table 1).30–32 Central reviewed response rate of nivolumab was 17% (95% confidence interval [CI] 10–28), median OS was 10.78 months (95% CI 7.4–13.3), and median progression-free survival (PFS) was 1.5 months (95% CI 1.4–2.8). The most frequent treatment-related adverse events of grade 3 or greater were decreased appetite (3%), lung infection (3%), increased blood creatinine phosphokinase (3%), and dehydration (3%); there were no treatment-related deaths. The ATTRACTION-1 trial showed that nivolumab monotherapy had promising efficacy and induced manageable adverse events in patients with metastatic or recurrent ESCC.30
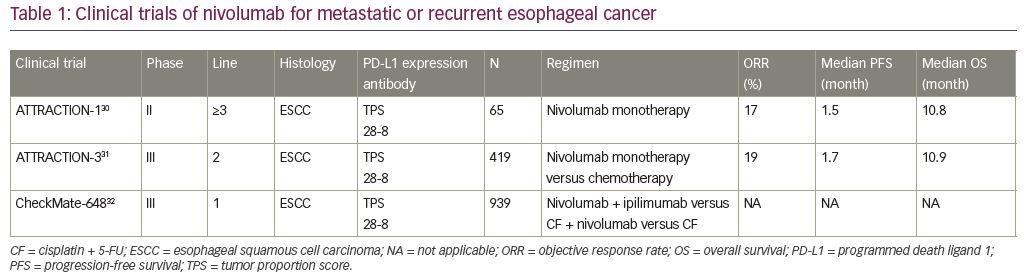
The phase III ATTRACTION-3 trial was an open-label, multicenter study comparing nivolumab monotherapy (240 mg every 2 weeks) with chemotherapy (paclitaxel 100 mg/m2 once per week for 6 weeks then 1 week off, or docetaxel 75 mg/m2 every 3 weeks) for ESCC or adenosquamous carcinoma.31 This study included 419 patients with metastatic or recurrent ESCC who were refractory or intolerant to previous fluoropyrimidine-based and platinum-based chemotherapy with either measurable or non-measurable lesions. After randomization, 210 patients were assigned to the nivolumab group and 209 to the chemotherapy group (Table 1).
Nivolumab monotherapy significantly improved OS compared to taxane (median OS 10.9 [95% CI 9.2–13.3] months versus 8.4 [95% CI 7.2–9.9] months, respectively; hazard ratio [HR] 0.77 [95% CI 0.62–0.96]; p=0.019). The response rates for the nivolumab and chemotherapy groups were 19% (95% CI 14–26) and 22% (95% CI 15–29), and the median durations of response were 6.9 (95% CI: 5.4–11.1) months and 3.9 months (95% CI 2.8–4.2); the median durations for PFS were 1.7 months (95% CI 1.5–2.7) and 3.4 months (95% CI 3.0–4.2), respectively. Additionally, there was no specific tendency of OS in the subgroup analysis based on PD-L1 expression evaluated by TPS. In the nivolumab group, the frequent treatment-related adverse events were rash (11.0%), diarrhea (10.5%), decreased appetite (7.7%), and fatigue (7.2%). Additionally, serious immune-related adverse events in the nivolumab group were diabetic ketoacidosis (one patient), intestinal lung disease (one patient), and pneumonitis (one patient).
The phase III ATTRACTION-3 phase III trial, which included previously treated patients with advanced ESCC, demonstrated that nivolumab was superior to taxane in terms of survival benefit and safety profile.31 Based on the results of the ATTRACTION-1 and ATTRACTION-3 trials, nivolumab was approved by the FDA on June 10, 2020 for patients with advanced esophageal cancer whose tumors are refractory to pyrimidine fluoride and platinum-based drugs.
Nivolumab monotherapy as an adjuvant treatment for patients with esophageal cancer with resectable tumors
The CheckMate 577 (ClinicalTrials.gov Identifier: NCT02743494) phase III trial was a randomized, multicenter, double-blind study that compared nivolumab monotherapy to placebo as the adjuvant treatment after complete resection of resectable esophageal cancer or esophagogastric junction tumors in patients who received neoadjuvant chemoradiation and in whom a pathologic complete response was not achieved. This trial demonstrated that nivolumab conferred significant survival benefits.33
In this trial, 794 patients were randomized to nivolumab (n=532; nivolumab 240 mg, every 2 weeks for 16 weeks, followed by nivolumab 480 mg every 4 weeks) or placebo (n=262). Approximately 70% of patients had esophageal adenocarcinoma and 30% of patients had ESCC. A pre-specified interim analysis showed that adjuvant nivolumab monotherapy conferred a statistically significant improvement in disease-free survival (DFS) when compared to placebo (median DFS 22.4 months [95% CI 6.6–34.0] versus 11.0 months [95% CI 8.3–14.3]; HR 0.69 [95% CI 0.56–0.86]; p=0.0003). Histological subgroup analysis showed that the median DFS of nivolumab-treated and placebo-treated patients with ESCC was 29.7 months and 11.0 months (HR 0.61), respectively. This improvement was generally superior to that of the median DFS of patients with esophageal adenocarcinoma (19.4 months versus 11.1 months; HR 0.75). The most common grade 3/4 treatment-related adverse events in the nivolumab group were pneumonitis (0.8%) and rash (0.8%); no treatment-related deaths were reported.33
The results of this trial suggest that adjuvant nivolumab monotherapy might be a new standard treatment for patients with esophageal cancer treated with neoadjuvant chemoradiotherapy followed by surgery that failed to achieve a pathological complete response.
Other immune checkpoint inhibitors for patients with advanced esophageal cancer
Pembrolizumab is a human monoclonal antibody that targets PD-1. The phase II KEYNOTE-180 trial study evaluated the efficacy and safety of pembrolizumab. The trial enrolled 121 patients; 63 (52%) had ESCC and 58 (48%) were PD-L1 positive. Tumors in patients were defined as PD-L1-positive if they had a CPS ≥10 following immunohistochemical staining with 22C3 antibody. CPS was defined as the number of PD-L1 positive cells (tumor cells, macrophages, and lymphocytes) divided by the total number of tumor cells, multiplied by 100.34
The primary endpoint of objective response rate was 9.9% (95% CI 5.2–16.7) for all patients, 14.3% (95% CI 6.7–25.4) for patients with ESCC, 5.3 (95% CI 1.1–14.4) for patients with esophageal adenocarcinoma, 13.8% (95% CI 6.1–25.4) for patients with CPS ≥10, and 6.3% (95% CI 1.8–15.5) in CPS <10; the median duration of response was not reached. In this trial, frequent treatment-related grade 3/4 adverse events were type 1 diabetes (3%), and pneumonia (2%). Pneumonia was the single grade 5 treatment-related adverse event.34
After the promising results of the KEYNOTE-180 trial, the phase III KEYNOTE-181 trial compared pembrolizumab monotherapy (200 mg every 3 weeks) with chemotherapy (paclitaxel 80–100 mg/m2 on days 1, 8, 15, every 4 weeks; docetaxel 75 mg/m2, every 3 weeks; or irinotecan 180 mg/m2, every 2 weeks) as second-line treatment for metastatic or recurrent esophageal cancer. The primary endpoints were OS in all patients, OS for patients with PD-L1 CPS ≥10, and OS for patients with ESCC. In this trial, 628 patients were randomized 1:1 to pembrolizumab or an investigator-selected regimen. Compared with chemotherapy, treatment pembrolizumab monotherapy of 222 patients with esophageal cancer with PD-L1 CPS ≥10 improved OS (median OS 9.3 months [95% CI 6.6–12.5] versus 6.7 months [95% CI 5.1–8.2]; HR 0.69 [95% CI 0.52–0.93] p=0.0074). On the other hand, pembrolizumab monotherapy did not improve OS in 401 patients with ESCC (median OS 8.2 months [95% CI 6.7–10.3] versus 7.1 months [95% CI 6.1–8.2]; HR 0.78 [95% CI 0.63–0.96]; p=0.0095) or in all 628 patients (median OS 7.1 months [95% CI 6.2–8.1] versus 7.1 months [95% CI 6.3–8.0]; HR 0.89 [95% CI 0.75–1.05], p=0.056).35,36
Based on the results of these two KEYNOTE trials, the FDA approved pembrolizumab in 2019 specifically for the treatment of patients with advanced ESCC with PD-L1-positive tumors (CPS ≥10) and in whom disease has progressed following at least one previous systemic treatment.
KEYNOTE-590 was a randomized, blinded, phase III trial that compared pembrolizumab (200 mg every 3 weeks for ≤35 cycles) plus chemotherapy (5-FU 800 mg/m2 for days 1–5 every 3 weeks for ≤35 cycles + cisplatin 80 mg/m2 every 3 weeks for ≤6 cycles) with chemotherapy alone (placebo + 5-FU 800 mg/m2 for days 1–5 every 3 weeks for ≤35 cycles + cisplatin 80 mg/m2 every 3 weeks for ≤6 cycles) for metastatic or recurrent esophageal adenocarcinoma, ESCC, and esophagogastric junction cancer. This trial randomized 274 (73.5%) patients with ESCC to pembrolizumab plus chemotherapy and 274 (72.9%) patients with ESCC to chemotherapy.37
Results of KEYNOTE-590 showed that pembrolizumab plus doublet chemotherapy was superior to doublet chemotherapy in terms of OS and PFS. The median OS in patients with ESCC with CPS ≥10 was 13.9 months (95% CI 11.1–11.7) versus 8.8 months (95% CI 7.8–10.5) (HR 0.57 [95% CI 0.43–0.75]; p<0.0001), in patients with ESCC was 12.6 months (95% CI 10.2–14.3) versus 9.8 months (95% CI 8.6–11.1) (HR 0.72 [95% CI 0.6–0.88]; p<0.0006), in patients with CPS ≥10 was 13.5 months (95% CI 11.1–15.6) versus 9.4 months (95% CI 8.0–10.7) (HR 0.62 [95% CI 0.49–0.78]; p<0.0001), and in all patients was 12.4 (95% CI 10.5–14.0) months versus 9.8 months (95% CI 8.8–10.8) (HR 0.73 [95% CI 0.62–0.86]; p<0.0001). The median PFS in patients with ESCC was 6.3 months (95% CI 6.2–6.9) versus 5.8 months (95% CI 5.0–6.1) (HR 0.65 [95% CI 0.54–0.78]; p<0.0001), in patients with CPS ≥10 was 7.5 months (95% CI 6.2–8.2) versus 5.5 months (95% CI 4.3–6.0) (HR 0.51 [95% CI 0.41–0.65]; p<0.0001), and in all patients was 6.3 months (95% CI 6.2–6.9) versus 5.8 months (95% CI 5.0–6.0) (HR 0.65 [95% CI 0.55–0.76]; p<0.0001). The secondary endpoint of objective response rate was also significantly improved (45% [95% CI 39.9–50.2] versus 29.3% [95% CI 24.7–34.1]; p<0.0001). Immune-related grade 3 or higher adverse events were reported in 7% of patients in the pembrolizumab plus doublet chemotherapy group.37 The results of the KEYNOTE-590 trial suggest that pembrolizumab plus doublet chemotherapy could be a new standard first-line treatment in patients with esophageal cancer with metastatic or recurrent disease.
Camrelizumab is a fully-humanized, selective IgG4-κ monoclonal antibody against PD-1. The ESCORT trial was a randomized, open-label, phase III study conducted at 43 centers in China.38 In this trial, 457 patients with ESCC were randomized to either camrelizumab (n=229) or chemotherapy (n=228). Median OS was 8.3 months (95% CI 6.8–9.7) in the camrelizumab group versus 6.2 months (95% CI 5.7–6.9) in the chemotherapy group (HR 0.71 [95% CI 0.57–0.87], two-sided p=0.0010), demonstrating that camrelizumab monotherapy was superior to chemotherapy when used as a second-line treatment. In the camrelizumab group, frequent grade 3 or higher adverse events were anemia (3%) and liver dysfunction (2%), and there were seven camrelizumab-related deaths (three unknown causes, one enterocolitis, one liver dysfunction, one pneumonitis, and one myocarditis). Therefore, camrelizumab may become a standard option for second-line treatment for patients with ESCC in China.38
Ongoing clinical trials of immune checkpoint inhibitors for patients with esophageal cancer
There are several ongoing phase III trials that are focused on patients with metastatic or recurrent esophageal cancer. The phase III CheckMate 648 trial, to compare nivolumab plus doublet chemotherapy or nivolumab plus ipilimumab with doublet chemotherapy as a first-line treatment for untreated patients with metastatic or recurrent ESCC (ClinicalTrails.gov Identifier: NCT03143153),32 has finished recruiting and is awaiting analysis. Tislelizumab (BGB-A317) is an anti-PD-1 antibody that was developed for the treatment of patients with metastatic or recurrent ESCC in China. The phase III RATIONALE-302 trial (ClinicalTrails.gov Identifier: NCT03430843) is a randomized study comparing the efficacy of tislelizumab with chemotherapy (taxane or irinotecan) as second-line treatment in patients with metastatic or recurrent ESCC. This trial has also finished recruiting and is awaiting analysis. In addition, the phase III RATIONALE-306 trial, to compare tislelizumab plus chemotherapy (5-FU plus platinum or paclitaxel plus platinum) with chemotherapy as a first-line treatment for untreated patients with metastatic or recurrent ESCC, is recruiting (ClinicalTrails.gov Identifier: NCT03783442).
Based on the results of the phase III PACIFIC trial,39 there are ongoing phase II and phase III trials in patients with unresectable, locally advanced esophageal cancer to evaluate the efficacy of definitive chemoradiation followed by an immune checkpoint inhibitor. The phase II NOBEL trial will evaluate the efficacy and safety of 5-FU- and cisplatin-based definitive chemoradiation plus nivolumab followed by sequential nivolumab monotherapy (UMIN ID: UMIN000035889) (Table 2),40,41 and the phase II TENERGY trial will evaluate the efficacy and safety of definitive chemoradiation followed by sequential atezolizumab (anti-PD-L1 antibody) monotherapy (UMIN ID: UMIN000034373).40 For inoperable, locally advanced esophageal cancer, patients are being recruited to the phase II CRUCIAL trial to evaluate efficacy and safety of FOLFOX-based definitive chemoradiation plus nivolumab followed by sequential nivolumab monotherapy, and chemoradiation plus nivolumab and ipilimumab followed by sequential nivolumab and ipilimumab (ClinicalTrails.gov Identifier: NCT03437200) (Table 2). Patients are also being recruited to the phase III KEYNOTE-975 trial that will evaluate the efficacy and safety of 5-FU- and cisplatin- or FOLFOX-based chemoradiation plus concurrent pembrolizumab monotherapy (ClinicalTrails.gov Identifier: NCT04210115).
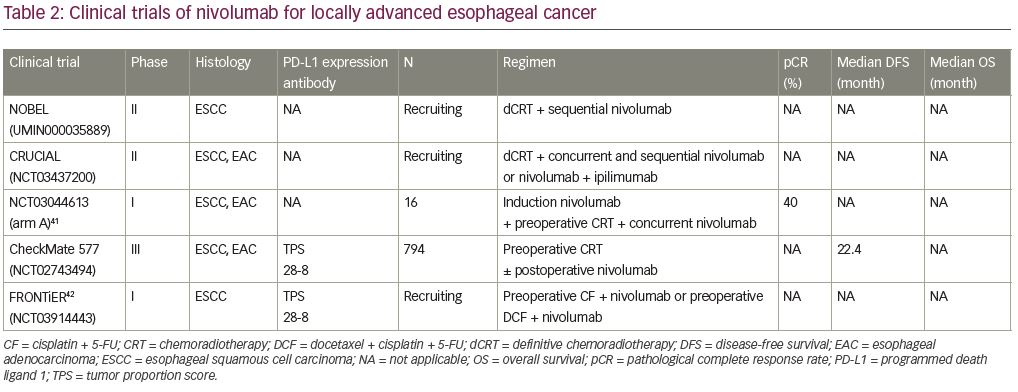
Finally, some phase I and phase II trials that focused on patients with resectable, locally advanced esophageal cancer have now reported their findings. A phase I trial to evaluate the safety and efficacy of induction nivolumab monotherapy and neoadjuvant chemoradiation plus nivolumab followed by surgery for patients with resectable esophageal cancer or gastroesophageal cancer has been conducted. In 16 patients, treatment-related adverse events occurred in 12 patients (75.0%), and 4 patients (25.0%) showed grade 3 adverse events (dyspnea and upper respiratory tract infection, transaminitis, and rash). Fourteen patients (87.5%) received all planned doses of nivolumab. The pathological complete response rate was 28.6% (4/14) for patients with esophageal adenocarcinoma, and 50.0% (1/2) for patients with ESCC.41 A phase II trial to evaluate the efficacy and safety of neoadjuvant chemoradiation plus pembrolizumab followed by surgery and adjuvant pembrolizumab monotherapy for patients with resectable ESCC (clinical T1N1-2M0, clinical T2-4aN0-2M0) has been conducted. The pathological complete response rate was 23.1% (6/26). In all 28 patients, 3 died during the preoperative period (massive hemorrhage) and after surgery (acute lung injury).43
The phase II PERFECT trial evaluated the efficacy and safety of neoadjuvant chemoradiation plus atezolizumab followed by surgery for patients with resectable esophageal adenocarcinoma. The completion ratio was 75% (30/40) and the pathological complete response was 37% (11/30). Immune-related adverse events of grade 3 rash (5%), grade 2 colitis (5%), and thyroiditis (5%) were reported.44 A phase I/II trial has also evaluated the efficacy and safety of preoperative chemoradiation plus avelumab (anti-PD-L1 antibody) followed by surgery and postoperative avelumab monotherapy for patients with resectable locally advanced esophageal cancer (clinical T1N1M0, clinical T2-3N0-2M0). The pathological complete response was 43% (3/7). Grade 2 hypothyroidism (5%) was the only immune-related adverse event reported.45 The above trials are developing treatments based on neoadjuvant chemoradiation. However, there is also an ongoing a phase I trial based on neoadjuvant chemotherapy (JCOG1804E/FRONTiER) that will evaluate the safety and efficacy of nivolumab plus 5-FU and cisplatin or nivolumab plus docetaxel and 5-FU, cisplatin for patients with resectable, locally advanced ESCC (ClinicalTrails.gov Identifier: NCT03914443)42 (Table 2).
Efficacy of immune checkpoint inhibitors by histology
The current article focused on the efficacy of nivolumab for patients with ESCC, the efficacy of immune checkpoint inhibitors might be different between ESCC and esophageal adenocarcinoma. In the ATTRACTION-130 and ATTRACTION-3 trials,31 only patients with ESCC were included. In the ATTRACTION-3 trial, OS for patients with ESCC was reported at HR 0.77 (95% CI 0.62–0.96).31 On the other hand, in the KEYNOTE-180 and KEYNOTE-181 trials, patients with ESCC and those with esophageal adenocarcinoma were included. In the subgroup analysis of the KEYNOTE-181 trial, OS for patients with ESCC and esophageal adenocarcinoma was reported at HR 0.77 (95% CI 0.63–0.96) and HR 1.12 (95% CI 0.85–1.47), respectively; and in the subgroup analysis of the KEYNOTE-590 trial,37 OS for patients with ESCC and esophageal adenocarcinoma patients were reported at HR 0.72 (95% CI 0.60–0.88) and HR 0.74 (95% CI 0.54–1.02), respectively. Additionally, in a subgroup analysis of the CheckMate 577 trial,33 HR for DFS in patients with ESCC and esophageal adenocarcinoma were reported at 0.61 and 0.75, respectively.
In consideration of these data, the efficacies of immune checkpoint inhibitors tend to be numerically lower in adenocarcinoma compared to ESCC, regardless of palliative and adjuvant treatments; however, at present, the difference is unclear. The biological mechanism of histological difference in response to immune checkpoint inhibitors has not yet been revealed. Therefore, further biological studies are needed to select appropriate subgroups for immune checkpoint inhibitors and to overcome tolerance for immune checkpoint inhibitors.
Conclusion and future perspective
Based on the results of the ATTRACTION-3 trial, nivolumab became the standard option for second-line treatment of patients with metastatic and recurrent ESCC regardless of PD-L1 expression.31 On the other hand, based on the results of the KEYNOTE-181 trial, pembrolizumab has also been adopted as the second-line treatment for patients with metastatic or recurrent ESCC with CPS ≥10.35, 36 Given access to second-line treatment for patients with advanced ESCC, nivolumab might be easier to administrate than pembrolizumab. However, the KEYNOTE-590 trial showed superiority of pembrolizumab plus doublet chemotherapy compared with doublet chemotherapy as first-line treatment for metastatic or recurrent patients with esophageal cancer.37 Therefore, pembrolizumab might be used more frequently as a first-line treatment and nivolumab monotherapy might be used less frequently as a second-line treatment as long as there is no evidence of immune checkpoint inhibitor continuation beyond progression. However, depending on the results of the CheckMate 648 trial, nivolumab plus ipilimumab or nivolumab plus doublet chemotherapy might be one of the standard treatments (ClinicalTrails.gov Identifier: NCT03143153).32
On the other hand, adjuvant nivolumab monotherapy may be used more frequently for patients with resectable esophageal cancer who received neoadjuvant chemoradiation followed by surgery, and those in whom a complete response was not achieved. In addition to neoadjuvant chemoradiation followed by surgery, neoadjuvant chemotherapy followed by surgery is increasingly used as a standard treatment. Therefore, further research into the efficacy of immune checkpoint inhibitors in the context of preoperative chemotherapy and surgery is required.












