The inhibition of angiogenesis and other signalling pathways has great potential in the treatment of various cancers including metastatic colorectal cancer (mCRC) and hepatocellular carcinoma (HCC). This approach is exploited by multikinase inhibitors (MKIs), which, when used in sequence with other chemotherapy agents, have shown to have efficacy with mostly manageable safety profiles although side effects with some of these treatments can be an issue. In the management of mCRC, patients are given widely differing chemotherapy combinations and sequences. The strategy for deriving optimal efficacy from these various agents, however, is not always clear.1 It is therefore worthwhile to explore the data supporting the use of one of these drugs, regorafenib, in this indication and in cancers of gastrointestinal (GI) origin. It is also worthwhile to consider the optimal sequencing of agents, particularly in third-line treatment and the future of combination strategies especially as there is a lack of clinical trial evidence on treatment sequencing.
HCC has proven difficult to treat, particularly in more advanced disease. Recent positive findings with regorafenib in HCC therefore are notable and indicate the potential to stabilise tumours and extend survival (the survival benefit was 2.8 months over placebo) in patients whose prognosis would otherwise be bleak.2 Since many physicians throughout the world have limited experience using the newer MKIs in either mCRC or HCC, there is an educational need to discuss their benefits

and their place in treatment strategies. This article therefore reports the presentations and discussions that took place at a satellite symposium on optimizing clinical outcomes in GI cancers through the inhibition of angiogenesis and other signalling pathways that was convened at the World Congress of Gastrointestinal Cancer at Barcelona in July 2016.
Evolving measures of efficacy in third-line metastatic colon cancer mCRC is a complex disease, and problematic for clinicians when patients become refractory to treatment. Patients with mCRC are most likely to have been pre-treated and have received both chemotherapy and monoclonal antibody treatments. In such patients, the critical issue is what should be given next? Can you still impact survival with third-line treatment? Refractory disease is unlikely to be caused by a single mutation and consequently it could be advantageous for treatment to adopt a ‘poly-pragmatic’ approach i.e., one in which drugs affect multiple different targets such as is the case with the MKIs.3–5
Regorafenib in metastatic colon cancer treatment
The MKI, regorafenib (Stivarga®, Bayer AG, Leverkusen, Germany) has multiple modes of action: it blocks the activity of various protein kinases that are necessary for angiogenesis, oncogenesis and the tumour microenvironment.5 Regorafenib has been investigated for the treatment of mCRC in two international, multicentre pivotal phase III randomised controlled studies: the Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer study (CORRECT, n=760)6 and the Regorafenib Plus Best Supportive Care Versus Placebo Plus Best Supportive Care in Asian patients with Previously Treated Metastatic Colorectal Cancer study (CONCUR, n=204);7 the latter included only Asian locations and populations. Importantly, in CORRECT, patients were treated with a single drug and there was an inclusion criterion for previous chemotherapy plus monoclonal antibody treatment whereas in CONCUR this did not apply. In CORRECT, treatment with regorafenib 160 mg resulted in a 23% reduction in the risk of death (primary endpoint) compared with placebo (median 6.4 versus 5.0 months p=0.0052) and in CONCUR this reduction was 45% (median 8.8 versus 6.3 months, p=0.00016).6,7 In addition, there was a 51% reduction in risk of progression or death in CORRECT compared with placebo (median 1.9 versus. 1.7 months, p<0.0001) and a 69% reduction in CONCUR (median 3.2 versus
1.7 months, p<0.0001). These results indicate that regorafenib has pronounced efficacy in mCRC.
In both the CORRECT and CONCUR studies, regorafenib appeared to provide greater benefits in less heavily pre-treated patients. This is supported by comparing the CORRECT study finding in which patients had all received previous targeted treatments, where the overall survival (OS) hazard ratio (HR) was 0.77, with the CONCUR study in which only 59% of patients had received previous targeted treatment and the OS HR was 0.55.7 Among patients in CONCUR, those with no targeted pre-treatment had substantially longer OS (HR = 0.31) compared with patients who had any targeted pre-treatment (HR = 0.78) (Figure 1).
Real-world data also demonstrate the benefits of regorafenib in mCRC. In the Regorafenib in Subjects with Metastatic Colorectal Cancer (CRC) Who Have Progressed After Standard Therapy (CONSIGN) phase IIIb trial (n=2,872) the median progression-free survival (PFS) was 2.7 months. This was broadly similar to the PFS determined in the CORRECT and CONCUR trials.8
Cavitation in metastases – likely related to treatment response
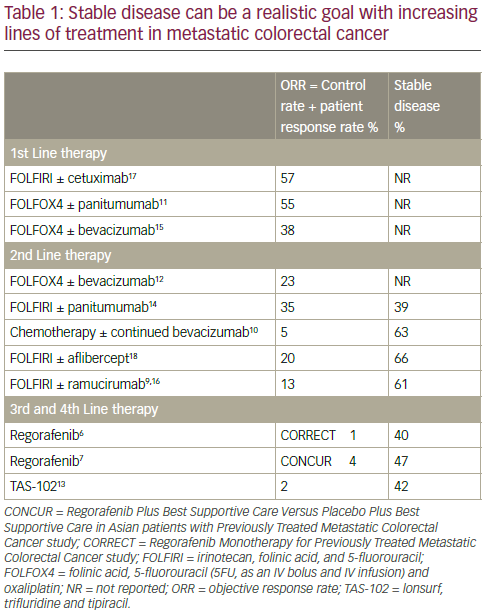
In mCRC treatment there is an evolution of expectation as the patient moves from first- to second- to third- and fourth-line treatments; therapeutic goals change as the treatment is altered in response to progressive disease. In particular, OS becomes more important than maintaining PFS; in advanced disease, few patients can be downstaged to surgery. When assessing the efficacy of first-, second-, third- and fourthline treatments in mCRC, it is also important to consider other endpoints including objective response rate (ORR) which is the sum of the response rate and the disease control rate (DCR). In third-/fourth-line mCRC treatment there is generally very little ORR observed (0–4%). However, disease stabilisation is achievable in 40–60% of these patients and this
is a very important benefit for some patients at an advanced disease stage (Table 1).6,7,9–18 Such disease stabilisation is often accompanied by morphological changes in primary tumours or in metastases and can sometimes be seen as cavitation. In this process, it is postulated that angiogenesis inhibition creates a central area of necrosis within the tumour.19,20 This was demonstrated in a retrospective evaluation of 108 patients in the CORRECT trail in which 29/75 (38.7%) regorafenib-treated patients and 0/33 (0%) of placebo-treated patients showed cavitation in lung metastases after 8 weeks of treatment (p<0.01).21 Among 60.9% of patients with cavitation in metastases, there was no response as determined by the Response Evaluation Criteria In Solid Tumors (RECIST) evaluation. The correlation between this cavitation and clinical benefit is under active investigation.19,20 In a subgroup of 75 patients with metastases, cavitation, lower density, shrinkage and RECIST response were all associated with longer PFS and OS.22
Predicting treatment response Among patients with mCRC there is a subgroup that responds well to regorafenib treatment. This good response (>4 months PFS) was shown by 19.4% in the CORRECT trial in which the median PFS for regorafenib was 1.9 months versus 1.7 months for placebo (p<0.0001).23 Similar findings were made in the CONSIGN real-world trial in which PFS was seen in 15% of patients at 6 months and 8% at 9 months.8 An analysis of the CORRECT trial data showed that an Eastern Cooperative Oncology Group (ECOG) status of 0 or 1, tumour metastases at ≤2 sites, time from diagnosis ≥18 months and treatment modifications were all factors that were apparently correlated with longer PFS (>4 months).23 Although not supported by statistical analysis, these observations suggest that it may be important to select mCRC patients with otherwise good general health and who have not been heavily pre-treated since they are more likely to show a good response to regorafenib.
The importance of biomarkers in metastatic colon cancer Biomarkers are increasingly important in cancer detection and monitoring. A study conducted at two treatment centres in Japan (n=121) determined that >10% decreases in levels of the carbohydrate antigen 19–9 (CA19–9) and >10% decreases in carcinoembryonic antigen (CEA) were associated with longer PFS after 28 days of regorafenib treatment (with a univariate analysis HR = 0.422, p=0.002 and HR=0.570, p=0.012, respectively).24 In patients with a >10% CA19–9 decrease, the median PFS was 3.7 months compared with 2.0 months in patients with <10% CA19– 9 decrease. This finding was as expected since CA19–9 is a biochemical marker of tumour response.
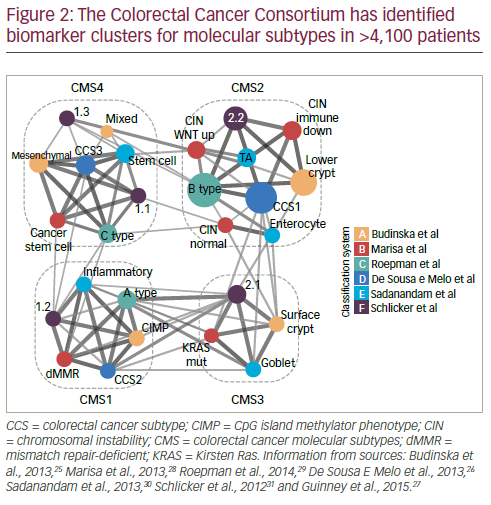
In order to resolve inconsistencies between reported gene expressionbased CRC classifications, an international consortium was established. This has used biomarker information from >4,100 patients which has been clustered according to molecular subtypes. This work has identified five main subtypes; four with distinct patterns (consensus molecular subtypes [CMS] 1 to 4) and another which cannot be classified into the other four (Figure 2).25–31 The CMS grouping is strongly related to patient prognosis: in one study (n=1,785) CMS4 was shown to be associated with much poorer relapse-free survival over 72 months than CMS1–3 (p≤0.0002 for all comparisons).27 In the same study, survival duration after relapse over 48 months was shorter with CMS1 and longer with CMS2 (p<0.0001); survival for those with CMS3 and CMS4 types was between the two.
The value of CMS grouping was highlighted in an analysis of the CORRECT study in which patients with a CMS2 or CMS4 molecular subtype (n=140 and 85) showed better OS and PFS when treated with regorafenib monotherapy (HRs for OS: CMS2 = 0.779; CMS4 = 0.672) than patients with CMS1 and CMS3 subtypes (HRs for OS: CMS1 = 1.116; CMS3 = 1.047).32,33 Patients with CMS3, however, showed the best PFS (HR = 0.287). These are notable findings suggesting that CMS subtyping could be a valuable tool for predicting a regorafenib treatment response at least in terms of OS. Further such analysis of biomarkers associated with treatment response in mCRC is needed to help delineate patients who would be suitable for particular treatments.
• Regorafenib has demonstrated improvement in survival and outcomes in geographically diverse mCRC patient populations
• Stable disease is a rational therapeutic goal in third-line mCRC because no better goals can be achieved
• Cavitation of lung metastases may provide additional insights into regorafenib antitumor activity
• Use of clinical measures such as ECOG status may help identify of mCRC patients who will benefit most from treatment with regorafenib although patients with a poor ECOG status may not tolerate this treatment
• Molecular markers may indicate potential regorafenib benefit and are under active investigation
Optimal sequencing of agents in third-line metastatic colorectal cancer – A case-based discussion
Receiving multiple therapies extends survival in metastatic colon cancer
Many of the improvements in mCRC survival seen over the past decade have resulted from advances in second-line and later therapies involving more active agents.15,17,34–37 It must be stressed that median OS in mCRC increases with exposure to more lines of therapy and therefore, patients should be expected to receive a series of different treatment regimens. A recent study (n=5,129) showed that moving from 0 to >3 total lines of therapy resulted in a corresponding increase in median OS of 6.8 to 26.4 months (HR changed from 0.604 to 0.364, p<0.001 for all comparisons).38 The choice of chemotherapy agent/biological agent combinations used as first- or second-line can be variable and is under investigation but the patients should receive different lines of therapy. However, reintroduction of chemotherapy beyond second-line without an interim treatment change is unlikely to have any survival benefit.39 Regorafenib has a different mode of action and adverse event profile to chemotherapy agents so its use as a third-line therapy may be justified.6,13 The European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) mCRC guidelines recommended regorafenib or trifluridine/tipracil (TAS-102) as the third or fourth-line treatment regardless of RAS status.40–42
Retreating with chemotherapy and other therapies after regorafenib can be beneficial
Retreating patients with chemotherapy after regorafenib may be appropriate and could provide renewed efficacy in some cases.6,42 In the CORRECT trial, 26% of patients received cytotoxic therapy after regorafenib.6 Combined experience from the Mayo Clinic, MD Anderson Cancer Center and the University of Southern California showed that in a population of 173 regorafenib-treated patients, 37% (n=64) received postregorafenib therapy of which, 52% (n=33) received standard treatment and 48% (n=31) entered first-line clinical trials with poor outcomes.43 Among the 33 patients receiving standard treatment, 20 (61%) showed an overall response or stable disease (eight had treatment previously discontinued without progressive disease, four had rechallenge with treatment discontinued due to progressive disease and eight had new treatment). The median OS among these patients following regorafenib discontinuation was 6.5 months.
A further treatment that offers clinical benefit in some patients after regorafenib is TAS-102. A phase III study (n=800) showed similar efficacy of TAS-102 in terms of OS in patients who had previous regorafenib treatment and those who had not (OS HR = 0.69 in both groups).13 Paradoxically, patients who had received greater total numbers of prior regimens did better than those who had received fewer (OS HR for two prior regimens = 1.05, ≥4 prior regimens = 0.59). This finding may have a bearing on future therapy sequencing in mCRC patients. Sequencing of TAS-102 after regorafenib was investigated in a small study in Japan.44 Patients were either treated with regorafenib and then TAS-102 (n=16) or were on TAS-102 (n=27). This was followed by subsequent therapy involving best supportive care, radiotherapy, targeted agents, radiotherapy or experimental drugs (alternatively, in the group that started on TAS-102, 6/27 was subsequently given regorafenib). Patient status at baseline was slightly better in the group pre-treated with regorafenib but the difference was not marked. In terms of PFS, OS and longer OS (up to 800 days) there were significant advantages for patients who received regorafenib first (p=0.0025 and p=0.0002 for PFS and longer OS, respectively). Due to the small sample size in this study, it is difficult to draw firm conclusions but the results are encouraging. In another recent trial, 51 patients with mCRC showed similar PFS and OS when treated with regorafenib or TAS-102 (PFS = 2.6 months and 3.2 months and OS = 10.6 months and 9.7 months, respectively).45 In this study, the toxicity profiles for these treatments, however, were different.
Case example showing typical disease progression and treatment in metastatic colorectal cancer
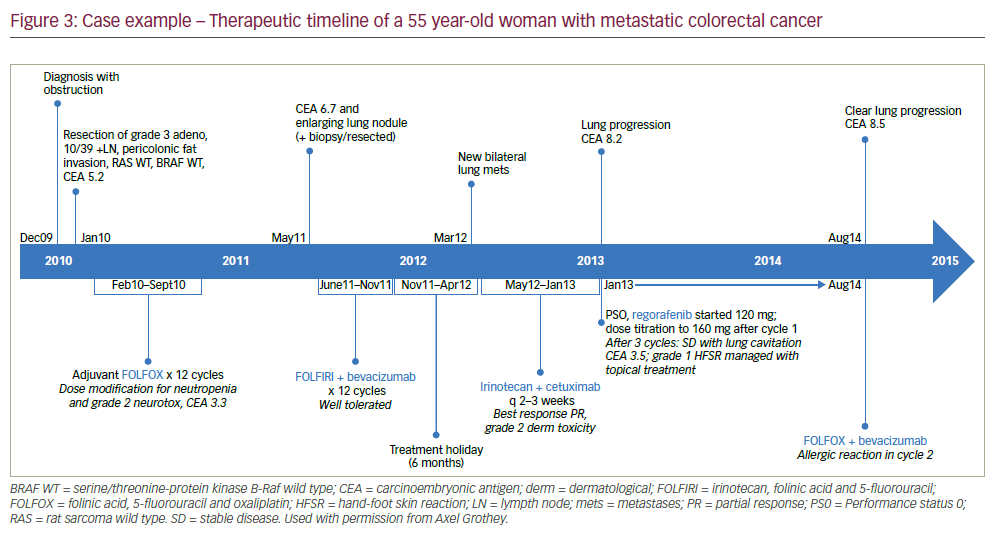
An mCRC case example of the treatment stages and changes in response to disease progression is given in the box below and summarised in Figure 3. This patient benefitted from regorafenib but also from TAS-102 as a following treatment stage. It is notable that 7 years after diagnosis and initial tumour resection, the patient has survived and still shows some disease control. Whilst this length of survival is impressive, this case emphasises the clear unmet clinical need for more effective treatments and combinations to better inhibit mCRC progression.
Case report
A 55 year-old woman was diagnosed with stage 3 colon cancer. After resection, she received 12 cycles of folinic acid, 5-fluorouracil and oxaliplatin (FOLFOX) over 8 months (see Figure 3 for a therapeutic timeline of this case). After a further 8 months, she had a relatively rapid recurrence (CEA 6.7 and enlarging lung nodule). After resection of the lung nodule she received 12 cycles of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) + bevacizumab for 6 months followed by a 6-month treatment holiday. This was followed by rapid disease progression (new lung metastases). She was then given irinotecan + cetuximab every 2-3 weeks for 7 months, from which she benefitted but then had further lung progression (CEA 8.2, Performance Status [PS] 0). She was started on regorafenib 120 mg which was titrated to 160 mg after one cycle. After three cycles, there was stable disease with lung cavitation (CEA 3.5). A grade 1 hand-foot skin reaction was managed with topical treatment. The patient received regorafenib for 8 months but then had lung lymph node metastases (CEA 8.5). At this point FOLFOX was recycled + bevacizumab but she had an allergic reaction in cycle two. She was consequently switched to TAS-102 that was newly available through an expanded access programme. Cycle 2 of TAS-102 was stopped due to neutropenia but treatment was restarted after symptom resolution.
Investigative combination treatment strategies may improve efficacy in metastatic colorectal cancer
At present, several combination strategies are being investigated for use in mCRC treatment. These include regorafenib + epidermal growth factor receptor (EGFR) inhibitors46 that may address resistance to EGFR blockade. One such ongoing study is investigating the safety of doses of cetuximab IV 200 or 150 mg/m2 with regorafenib 80 mg or 120 mg daily in 27 patients with locally advanced or metastatic tumours.47 Another combination, regorafenib + chemotherapy,48 has been investigated in a phase II study (n=181) comparing regorafenib + irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) versus placebo + FOLFIRI as secondline treatment in patients with mCRC.49 Median PFS and OS were 6.14 months and 13.2 months for regorafenib and 5.29 months and 12.0 months for placebo (p=0.02 for PFS and 0.76 for OS). The addition of regorafenib to FOLFIRI, was tolerable and significantly extended PFS. In addition, a planned phase IIb dose-finding study will assess PFS, tumour response, pharmacokinetics and safety of a regorafenib + TAS-102 combination.50 A further combination approach is to use vascular endothelial growth factor (VEGF) inhibitors + immunotherapy.51 This may provide a valuable strategy that exploits anti-tumour immune response along with various anti-tumour vascular effects. Initial studies suggest that this combination has potential in metastatic melanoma and may be of benefit in mCRC.51 The findings of these combination therapy studies are awaited with interest.
• Treatment options for mCRC remain a growing need – patients should be exposed to as many agents as possible to prolong OS
• Regorafenib is an evidence-based and guideline-recommended standard of care for pre-treated mCRC patients with good performance regardless of RAS status
• Patients may feasibly receive anti-tumour therapy for mCRC after regorafenib including TAS-102
• Combination strategies with regorafenib may further enhance anti-tumour activity and outcomes in mCRC although evidence so far is limited
Optimising clinical use of multikinase inhibitors in hepatocellular carcinoma
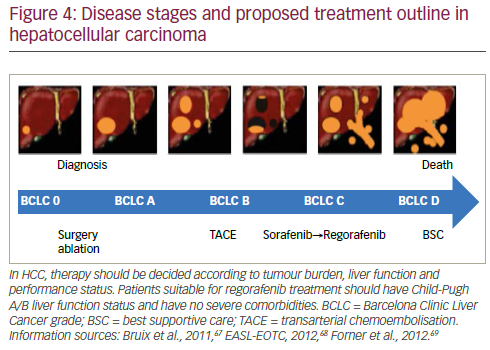
The treatment of HCC is likely to see substantial change in the near future as more effective therapy becomes available. Strategies adopted by leading specialist centres such as the Barcelona Clinic Liver Cancer (BCLC) follow the principle that diagnosis and management of this disease should be evidence-based.52 In current approaches, patients’ disease is categorised as: very early, early, intermediate, advanced and terminal stages. Ideally, during these stages patients are moved through different treatments starting with ablation followed by resection, transplant, further ablation, transarterial chemoembolisation, medications (including sorafenib) and, in the terminal phase, best supportive care. In reality however, few patients receive more than one or two treatment modalities, there is a very high drop-out rate and few receive systemic therapy. This was highlighted by the ‘Bridge to Better Outcomes in HCC’ (BRIDGE) study (n=18,031) that investigated global patterns of HCC management and found considerable heterogeneity in treatment and consequently varied survival rates worldwide.53
New treatments and combinations for hepatocellular carcinoma – early promise that was not fulfilled
Approximately 10 years ago, a considerable advance in HCC treatment resulted from the development of the kinase inhibitors including sorafenib sunitinib, linifinib, erlotinib and bivanib. In the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP, n=602)54 and Asia- Pacific (n=226)55 trials, sorafenib produced significant improvements in median survival time versus placebo (10.7 versus 7.9 months and 6.5 versus 4.2 months, respectively). Sorafenib also produced improvements in radiological progression and was tolerated by patients. These results were met with great enthusiasm and were thought to herald a new era in more successful HCC treatment. To investigate these agents in larger patient populations, various phase III trials were initiated using these kinase inhibitors in combination or with other drugs such as doxorubicin as first-line or second treatment approaches in HCC.56–64 Disappointingly, these trials failed to show any survival improvements; the early promise of these compounds and combinations in smaller trails was not substantiated in the larger studies.57,59
Regorafenib in hepatocellular carcinoma – a longawaited step toward improved outcomes
More recently, regorafenib has been evaluated in HCC. When used as a second-line treatment, regorafenib showed anti-tumour activity and a manageable safety profile in a phase II trial of patients (n=36) with BCLC grade B or C HCC whose disease had progressed during sorafenib treatment.65 Based on these findings, the phase III Regorafenib Versus Placebo in Patients with Hepatocellular Carcinoma (RESOURCE) trial was initiated.66 This study was conducted at 152 centres in 21 countries (in North and South America, Europe, Australia and Asia) and included only patients with documented radiological HCC progression during previous sorafenib treatment. Patients (n=573) were randomised 2:1 to regorafenib 160 mg once daily (OD) (3 weeks on/1 week off in a 4-week cycle) or placebo and were treated until disease progression, unacceptable toxicity or withdrawal. In this trail, patients were stratified according to geographic origin, macrovascular invasion, extrahepatic disease, ECOG status (0 versus 1) and alfa fetoprotein level (<400 ng/ mL versus ≥400 ng/mL). Patients were also required to have shown tolerability to prior sorafenib ≥400 mg daily for at least 20 of the last 2 8 days of treatment.
The population of the RESOURCE study were mostly male and around 60 years of age (44% from Asia) and the majority had BCLC grade C disease. The results have not yet been fully released but show that the study met the primary endpoint in that regorafenib treatment provided a statistically significant and clinically meaningful improvement in OS. There was a 38% reduction in risk of death (HR = 0.62, 95% confidence interval [CI] = 0.5–0.78; p<0.001). The median OS was 10.6 months versus 7.8 months and the survival benefit was maintained in all subgroups. Regorafenib treatment also significantly improved PFS and time to progression (TTP) and was maintained in all predefined subgroups. In addition, patients treated with regorafenib had a significantly higher response rate and almost doubled DCR. Adverse events were manageable and consistent with the known safety profile of regorafenib. These events include skin reaction, fatigue, hypertension and hyperphosphataemia. From observations made in this trial, the assertion, based on CRC studies that regorafenib is toxic to the liver, was rejected.66
The need for altered guidelines for assessing treatment efficacy in hepatocellular carcinoma
The reason why various agents and combinations for HCC treatment have failed in large trials despite positive earlier signals remains unknown. It is clear that for sorafenib and other drugs, the sets of assessments used did not adequately predict efficacy in larger trials and refinements of the parameters used or the use of new endpoints is needed for the determination of anticancer efficacy of future therapies. Currently, OS is the most important endpoint but PFS, TTP and DCR are frequently used. This set may need to be expanded to include tumour progression, response rate and other criteria.
In HCC, the guidelines for evaluating response are much more numerous than those of other cancers. This means that assessing response is more complex and the definitions and parameters used may vary between different studies and investigators. Response to treatment may not be the critical issue in HCC treatment; if drugs inhibit or prevent disease progression the patient will survive and this may be a more valid criterion for measuring treatment efficacy than existing measures of response.66 Therapy should be selected according to tumour burden, liver function and performance status. In view of the favourable results seen in the RESOURCE trial, current treatment guidelines may need to be adapted to incorporate regorafenib.35,67–69 This treatment has the potential to improve outcomes and become the standard of care for second-line treatment of HCC after progression of disease during sorafenib treatment as outlined in Figure 4.
• The promise of new kinase inhibitors such as sorafenib in early studies in HCC was not substantiated in larger phase III trials of these drugs used in combinations as first- or second-line therapy
• The reasons for failure of several treatments to improve survival after early favourable results may have been lack of efficacy, liver toxicity and the parameter sets used
• Regorafenib showed good results in early studies and interim results from the larger phase III RESOURCE trial were striking with significantly longer TTP, PFS and OS compared with placebo and a manageable safety profile
• In the clinic, regorafenib has the potential to markedly improve the prognosis in patients with HCC
• In future, efficacy assessments/guidelines for HCC may need to be expanded to include tumour progression, response rate and other criteria in addition to the currently used measures of TTP (reflects tumour progression), DRC (reflects response rate) and OS
Conclusion
In both mCRC and HCC, effective and long-lasting drug treatments remain serious unmet medical needs. The development of MKIs, particularly regorafenib, however, has brought renewed hope in both these cancer types and offers a new and effective option in advanced disease. In mCRC, as third-line treatment, regorafenib showed significantly improved survival over placebo.6,7 In both the CORRECT and CONCUR studies, patients had been heavily pre-treated and in the former, patients were given regorafenib as monotherapy. These findings were validated in realworld populations in the CONSIGN trial.8 Interestingly, very low rates of ORR are reported in various trials on mCRC treatment combinations but stable disease is achieved in 40–60%. This is an important finding which suggests that the criteria used to evaluate efficacy of treatments in advanced disease may need to be expanded to more accurately assess efficacy and predict disease control in larger patient populations. It is also notable that among a subgroup of in the CORRECT trial, cavitation was seen in 38.7% of regorafenib-treated patients but 0% in the placebo-
treated patients.21 In addition, most of those with such cavitation showed no RECIST response. This indicates that current assessment parameters may not capture all aspects of drug efficacy. Analyses of the CORRECT study also showed some potential for biomarkers such as CMS1–4 molecular subtypes, for predicting a response to regorafenib and identifying suitable candidates for treatment.32,33
The fact that improvements in mCRC survival have resulted from developments in second-line and later therapies15,17,34–37 indicates that more active compounds are reserved for more advanced disease. Patients benefit from receiving multiple different therapy combinations with differing modes of action and these must be altered as the disease progresses. This contention is supported by the finding that patients who received more lines of therapy had better OS than those with fewer.38 In addition, patients who receive regorafenib tend to perform better on subsequent therapies than those who have not received it.6,43 Patients who progress on regorafenib can benefit from a return to previous chemotherapy and certainly improve when switched to newer treatments such as TAS-10244 In mCRC, therefore, regorafenib is an evidence-based standard of care according to guidelines and its wider use is likely to limit tumour progression and consequently increase the duration of survival.
The mCRC case example illustrates the progression of a patient through the disease stages and the differing therapies that are likely to be used in current treatment strategies. It is notable that five years after first diagnosis, this patient is still receiving treatment. This indicates that whilst mCRC cannot be eliminated, it can be stabilised and exposing the patient to multiple different therapies including regorafenib and other new treatments at later stages, can extend survival for long periods.
It was disappointing that the early promising results with sorafenib and various drug combinations in HCC were not substantiated in phase III trials.56–64 This finding largely reflects the need for more effective treatments in HCC. It is also likely that the criteria used to judge efficacy in early trials were inadequate and over-estimated efficacy. In view of this, the guidelines for evaluating treatment success in HCC need to be expanded to include tumour progression and other parameters, in order to avoid emerging drug treatments not meeting expectations and further large-scale trials proving negative.
Against this background of repeated failure, the newly emerging results from the RESOURCE study are striking and provide new hope in advanced HCC.66 In all subgroups in a large and diverse population of patients who had progressed on sorafenib, treatment with regorafenib produced greatly improved OS, risk of death, TTP, PFS, response rate and DCR compared with placebo and other current treatments. Regorafenib was also well tolerated with a manageable safety profile. These findings are so important that they are likely to change current practice in HCC management. They may also influence ongoing trials of other HCC treatments since the ethics of using less effective treatments could become questionable. The final results of the RESOURCE study and subanalyses are awaited with interest. Use of regorafenib in combination with multiple other treatments in HCC is an intriguing prospect that may yield further efficacy gains.
In both mCRC and HCC the development of biomarkers and the use of improved diagnostic and treatment guidelines that specify an expanded set of criteria and endpoints are likely to improve and inform management of these diseases. In addition, the wider use of regorafenib and other newly emerging treatments for advanced disease is likely to improve the prognosis and substantially extend survival. It is, however, important that upon disease progression, treatment is promptly switched to regorafenib or other new agents before patients become too fatigued from previous treatment regimens to derive the full benefit.













