Regorafenib was approved as a third-line treatment for colorectal cancer in 2013. Notwithstanding its promising pharmacodynamics, 5 years after its introduction, only a small subgroup of patients has profited from this drug, and in those who did, the treatment effects were surprisingly restricted.1
Regorafenib is an oral multikinase inhibitor against vascular endothelial growth factor receptor 1 (VEGFR-1), VEGFR3, VEGFR1/3, platelet-derived growth factor receptors subunit beta (PDGFR-β), fibroblast growth factor receptor 1 (FGFR1), TIE-2c-KIT, RET/Raf1 and protein kinase B-RAF (BRAF); it has three targets: angiogenesis, oncogenesis and proliferations.2
The convenience of the administration route, plus its multitasking mechanism of action, could promise a new age in the treatment of many types of cancer.3 Also, regorafenib presents a tolerable set of side effects as compared to current cytotoxic and antiangiogenic therapies. Apart from the approval of regorafenib in advanced and inoperable gastrointestinal stromal tumours (GISTs), and the recent approval for the treatment in hepatocellular carcinoma (HCC) progressing after sorafenib treatment, the scope of prescriptions for regorafenib has remained disappointingly limited.4 All the approved treatments for regorafenib have one characteristic in common: it is always the last-line agent, and it is only effective in patients who have failed previous therapies.5 This subgroup of patients restricts the current usage of regorafenib in a triplet of highly lethal diseases, in highly advanced cases, and in short-survival patients, usually with other diseases. Some attempts have been made with regorafenib in earlier cancer, but with negative results, to date.6
Another aspect of regorafenib is cost–benefit. At present, it presents a high cost with very little or no benefit for patients, thus presenting an unfavourable cost–benefit relation.1 As for the future, regorafenib may present a positive synergic administration with checkpoint inhibitors, as it has been associated with bevacizumab.7
The aim of this review is to present the current and possible future usages of regorafenib, summarising the pharmacodynamic targets of this drug, and to pave way for new clinical trials that may benefit more patients in an acceptable cost–benefit ratio.
The literature review was conducted in the following steps: identification theme and formulation of the research, construction of an instrument for collecting relevant data from the articles found, evaluation and analysis of selected articles, and interpretation of results obtained. The searches were performed on the following bases of bibliographic data: PubMed, Science Direct and Scopus. The search criteria used were clinical phase I, II or III clinical trials; case reports; retrospective studies; and reviews in basic sciences published between 1992 and 2019, available in English and addressed the theme of the drug regorafenib. Sixty articles met the criteria drawn by the authors and were therefore included in the review.
Review of drug recipients for regorafenib
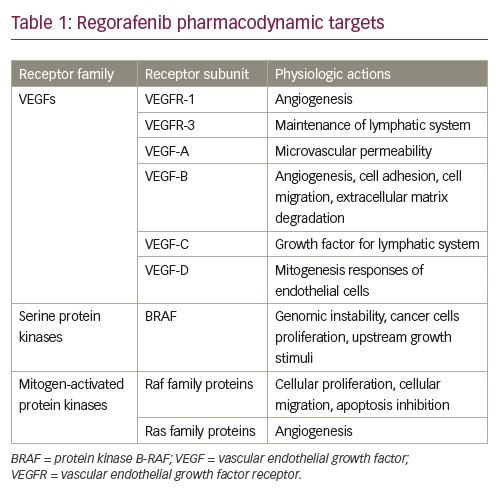
Vascular endothelial growth factors
VEGFs comprise a polypeptide family with multiple functions involved in angiogenesis, lymphangiogenesis and increased capillary permeability acting on specific receptors on cell surfaces.8,9 The VEGF family consists of five members (VEGF-A, VEGF-B, VEGF-C, VEGF-D and VEGF-E) that have their functions mediated by the tyrosine kinase receptors (RTK) VEGFR-1, VEGFR-2 and VEGFR-3.9,10 Intracellular phosphorylation initiates a cascade of cytoplasmic reactions that culminates in several cellular responses related to migration and proliferation of endothelial cells, as well as fenestration and permeabilisation of the vessel.11–14
VEGFR-1 is an RTK that plays a role in physiological and pathological angiogenesis in the context of receptor dimerisation and an interaction with its ligands.15 The VEGFR-1 receptor, found in the endothelial-cell membrane, when binding to the VEGF-A ligand promotes cell migration, blood vessel organisation and gene expression of monocytes and macrophages.16 VEGFR-3 is an RTK expressed on all endothelial cells during early embryogenesis. During later developmental stages, the expression of VEGFR-3 is restricted to lymphatic vessels.17,18 VEGFR-3 is therefore involved in the remodelling of primary capitis vasculature, embryonic cardiovascular development, and regulation of growth and maintenance of the lymphatic system.18,19 Embryonic defects related to the VEGFR-3 gene during the stage of development of vasculogenesis, leads to the formation of abnormal budding, organisation and vascular remodelling.18
VEGF-A (also known as vascular permeability factor) was originally purified based on its mitogenic activity towards endothelial cells, and on its ability to induce microvascular permeability.19 VEGF-B has angiogenic properties and may be involved in cell adhesion and migration and in the regulation of extracellular matrix degradation. VEGF-B is expressed primarily in cardiac and skeletal muscle tissues of the embryo and adults.10 VEGF-C was identified as a growth factor for the lymphatic vascular system. During embryogenesis, VEGF-C is expressed in a complex spatial and temporal pattern, and its expression persists in the heart, lung and skeletal muscles in adults. VEGF-D stimulates mitogenic responses in endothelial cells.19
Mitogen-activated protein kinase pathway
The mitogen-activated protein kinase (MAPK) pathway leads to cell proliferation, differentiation, migration, survival and angiogenesis.20 This pathway is composed of the small guanidine triphosphatase (GTPase), which activates the Raf family proteins.21,22 Ras, a small GTP-binding protein, is an important component of the signal transduction pathway used by growth factors to initiate cell growth and differentiation. Cellular activation with growth factors, such as epidermal growth factor (EGF), induces Ras to move from an inactive state linked to GDP to an active state linked to GTP.21
Recently, a combination of genetic and biochemical studies has resulted in the elucidation of a signalling pathway leading from growth factor receptors to Ras. Following EGF binding, the EGF tyrosine kinase receptor is activated, leading to autophosphorylation of the receptor in multiple tyrosine residues.21,23,24
BRAF
BRAF is a serine/threonine protein kinase, encoded on chromosome 7q34, which activates the MAPK/extracellular signal-regulated kinase (ERK) signalling pathway. BRAF is the family member most easily activated by Ras.25,26 In addition, BRAF basal kinase activity is higher than that of other family members.27,28 This provides a potential reason for the frequent mutational activation of BRAF seen in human tumours.29
Raf kinases can homo- and heterodimerize,30,31 and the structure of an active Raf kinase is that of a side-by-side dimer in which only one partner should have catalytic activity.32 Dimerization is improved by Ras33 and is subject to negative feedback regulation by ERK.31,34 Several Raf mutations have been implicated in the induction of genomic instability, boosting the proliferation of cancer cells more frequently in melanoma. For example, BRAF signals mutated as a monomer, independent of upstream growth stimuli. A more frequent BRAF mutation, BRAF V600E, provokes constitutive kinase, as well as insensitivity to negative feedback mechanisms.29,35 Table 1 summarises drug targets for regorafenib.
Current approved uses of regorafenib
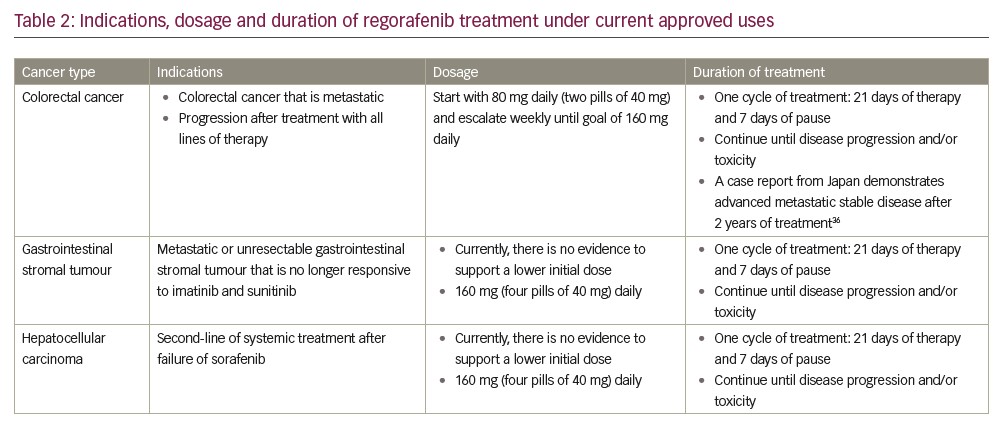
As of 2018, regorafenib was approved by the US Food and Drug Administration (FDA) for three specific clinical settings. The first approval of this tyrosine kinase inhibitor was for colorectal cancer and, in this scenario, after progression of standard adjuvant treatment in metastatic disease. It is advocated that treatment continues until disease progression or unacceptable toxicity levels have been reached, which in some cases, as demonstrated in a recent Japanese case-report, may take several years.36 Subsequently, approval was granted for management of GIST. Also, approval is almost prohibitively narrow, as it comprises a tumour that is either locally advanced, unresectable or metastatic after treatment with imatinib and sunitinib.36,37
Most recently, attention has been drawn to HCC. Similarly, to the two prior approvals, in this case, the scope of the drug is for patients who are candidates for systemic treatment as a second-line treatment after failure or intolerance for sorafenib. In this scenario, it is yet unclear whether regorafenib or the immune checkpoint inhibitor nivolumab is the most beneficial line of therapy.37 Dosages and indications are summarised in Table 2.38,39
Possible complications of therapy with regorafenib
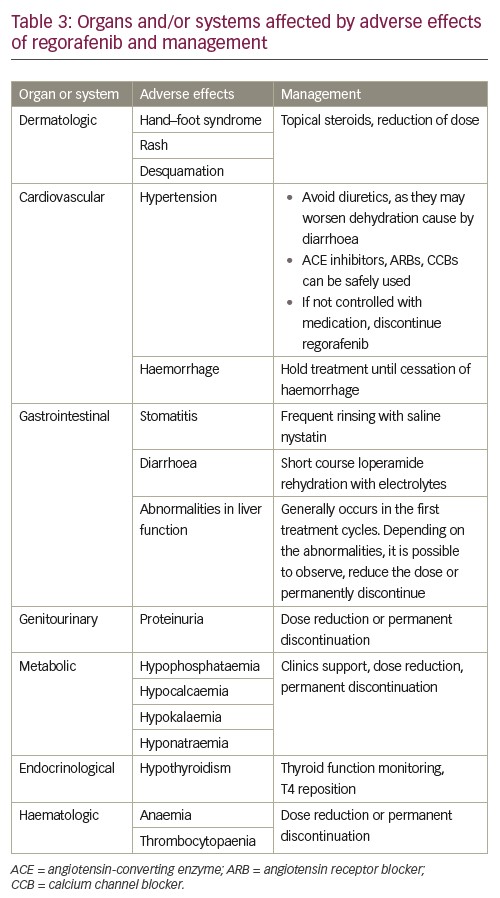
As is germane to every antineoplastic treatment currently available, the profile of side effects is crucial to patient adherence, completion of therapy and therapeutic success. In the particular case of regorafenib, notwithstanding the convenience of its oral presentation, serious side effects may occur, and make the continuation of therapy unadvisable. Most patients will present with a grade three or four adverse effects. According to the current evidence, the most frequent side effects are hand–foot syndrome, fatigue, diarrhoea, hypertension, thrombocytopaenia and anaemia.3
Interestingly, hand–foot syndrome has been reported, by some clinical trials, to be related with a better outcome. Although it is still early to conclude the causes or the magnitude of this relation, this piece of information may become a tool to predict outcomes of the treatment in the future.3 Also, hypothyroidism has been shown to be an independent predictor of improved overall survival in patients with colorectal cancer. A prospective study in Korea has demonstrated clinical hypothyroidism as a statistically significant protective factor for overall survival (hazard ratio 0.35; p=0.007).40 A study in Italy has drawn similar conclusions.41 Other side effects have been associated with improved progression-free survival in regorafenib monotherapy for advanced colorectal cancer. The TRIBUTE analysis was a retrospective study of 144 patients that demonstrated improved progression-free survival in the subsets of patients who developed skin rash, hypertension, diarrhea and hypothyroidism.42
Of note, severity of side effects differs between types of cancer.43 Treatment of metastatic colorectal cancer is related with more severe side effects than GIST and HCC. This might be related to the timing of therapy. Regorafenib therapy for GIST and HCC is approved after failure of other tyrosine kinases inhibitors, which means that patients have already been exposed to this class of drug, lessening potential side effects. The most important side effects are summarised in Table 3.
Survival benefit with regorafenib
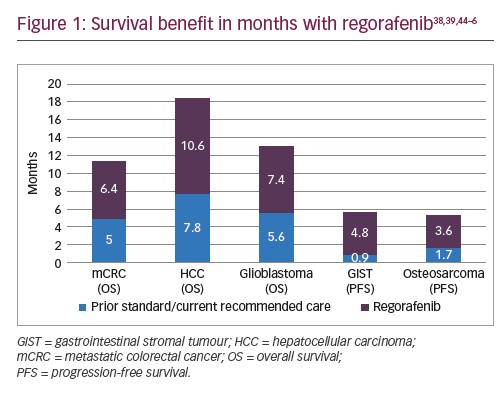
Regorafenib shows a survival benefit in a variety of cancers.38,39 Regarding the labelled indications, patients with metastatic colorectal cancer and advanced HCC may benefit from modest months increases in overall survival. As for GISTs, in spite of increase in progression-free survival, regorafenib has failed to improve overall survival.44 The latter may be due to the design of the study, allowing patients to crossover between the two arms of the trial. Similarly, it has been associated with prolonged progression-free survival in osteosarcoma,45,46 with no benefit in overall survival. Survival benefits with regorafenib compared to prior standard/current recommended care are summarised in Figure 1.
Future directions
Five years after its approval, regorafenib remains a drug with limited clinical handling. Approved use in colorectal cancer, GIST and HCC is only for advanced metastatic disease. Combined with high cost, there is currently little clinical benefit for patients. Moreover, distinct trials are being conducted in order to define it as a new treatment option.45,47,48 Future directions for this drug include the management of osteosarcoma. A recent placebo-controlled, double blind trial in France has shown an increase in progression-free survival by a factor of 3 in patients with metastatic osteosarcoma that have failed every line of treatment.45 Compellingly, these new data show benefit on advanced metastatic disease as a last resort, similarly to all current approved uses.
Recent data suggest a possible synergic effect between regorafenib and immune checkpoint inhibitors, as has been shown in the REGONIVO trial.47 A phase Ib trial comparing regorafenib and its combination with nivolumab in patients with advanced gastric cancer or colorectal cancer, demonstrated 38% objective response rate (44% in gastric cancer and 36% in colorectal cancer) and a tolerable side effects profile in the combination group. This intriguing benefit may be due to reduction of tumour-associated macrophages by regorafenib, increasing the tumour’s sensitivity to nivolumab. Currently, the REGONIVO phase II trial is under way and could soon corroborate this hypothesis. Additionally, a latter phase II clinical trial has demonstrated that regorafenib is superior to lomustine in advanced and relapsed glioblastoma.48 The REGOMA trial, in Italy, has indicated a significant improvement in overall survival (hazard ratio 0.50; 95% confidence interval 0.33–0.75; log-rank p=0.0009) as compared with lomustine therapy.48
The REVERSE studies have been conducted with regorafenib and cetuximab in the treatment of metastatic colorectal cancer. The results obtained on the sequence of the use of such drugs in the treatment of this cancer suggest that the ideal order would be the initial administration of regorafenib followed by cetuximab, different from the standard protocol currently used. The results showed improved overall survival of the patients and the benefit seemed to be driven mostly by greater activity of cetuximab than regorafenib as the second treatment.49,50
The INTEGRATE trial of regorafenib monotherapy in gastric cancer showed that this drug was well tolerated and that there was no damage in patients’ quality of life compared to those who received placebo and that it did not appear to have an excessively negative effect on those parameters from toxicity.51,52 Research projects highlighted that baseline levels of pain, appetite, constipation, and physical functioning were found to be significant prognostic factors for survival.53 Also, this trial demonstrated that regorafenib had considerable activity in the primary progression-free survival endpoint.54,55 Additionally, the phase II REDOS trial was performed from 2015–2018 and authors showed that a dose-escalation strategy for regorafenib is an achievable alternative to the standard regorafenib dosing strategy of 160 mg/day, especially in patients with metastatic colorectal cancer. It was also found that patients treated with dose escalation had a higher frequency of post-progression treatment and numerically longer overall survival.54,55
Regarding tolerability of regorafenib when employed to treat colorectal cancer, limited data are available on the tolerance in the older patient population, and the decision must be made considering the minimal survival benefit and the toxicity profile.56 Considering this drug in HCC treatment, research projects emphasize that there is an acceptable tolerance profile and that regorafenib provides a survival benefit.57 For GIST treatment, several authors state that regorafenib is well tolerated with no unexpected toxicities.58,59
Further research is needed to determine which patients can benefit the most from this drug. As of 2019, ongoing trials are testing whether regorafenib can improve outcomes in soft tissue sarcomas, such as osteogenic sarcoma, liposarcoma, Ewing sarcoma and rhabdomyosarcoma.45
Conclusion
Despite 5 years of approval and promising pharmacodynamics, regorafenib has shown limited, yet statistically significant, benefit for various types of solid tumours.60 Labelled indications comprise colorectal cancer, GIST and HCC. Advanced phase II trials have shown significant improvements in survival for gastric cancer, glioblastoma and osteosarcoma, which may indicate future inclusion in labelled indications.44–47
Combination therapy with immune checkpoint inhibitors has been demonstrated as beneficial in phase I trials, and phase II trials are being performed. Currently, regorafenib is being investigated for other cancers as well.7,61 Many individual side effects can be used as markers for better outcomes with treatment. Among those, hand–foot syndrome and hypothyroidism are the most related to improved survival. In summary, studies have shown that regorafenib can significantly improve survival with an acceptable tolerance in a variety of solid tumours.












