Targeting kinase pathways to treat progressive gastrointestinal stromal tumours and sarcomas
Gastrointestinal stromal tumours
Medications that target multiple kinase pathways have proven to be a successful and are a frequently used approach in treating progressive gastrointestinal stromal tumours (GISTs), which are the most common type of sarcomas.1–4 Tyrosine kinase inhibitors (TKIs), which have shown efficacy and are approved for use in GISTs include regorafenib, imatinib, sunitinib and pazopanib. Some of these, and other TKIs also have potential for the treatment of other cancers, including soft tissue sarcomas (STS) and bone sarcomas (BS).5–8
GISTs are caused mainly by KIT mutations in the tyrosine kinase gene at exon 11 (67.5%), exon 9 (11%), exon 13 (0.9%) and exon 17 (0.5%) and less frequently by platelet-derived growth factor receptor (PDGFRA) mutations at exon 18 (6.3%), exon 12 (0.9%) and exon 14 (0.3%).9 The efficacy of the TKI imatinib is dependent on the type of mutation present, for example, patients’ KIT mutations at exon 9 or 11 and PDGFRA exon 12 are imatinib sensitive.9,10 Findings from the GIST meta-analysis group have suggested that patients with KIT exon 9 mutations may have progressionfree survival (PFS) on a higher dose of imatinib (800 mg compared with 400 mg), but the analysis was insufficiently powered to make a definitive conclusion.11 Findings also show that the PDGFRA-D842V mutation is particularly resistant to imitanib treatment, whereas some other PDGFRA mutations are not resistant to this treatment.11,12 Patients with unresectable or metastatic GIST are very difficult to treat but imatinib is effective as first-line therapy in many such cases. A recent comparative study of patients with advanced GIST (ENESTg1) showed that in those with KIT exon 9 mutations, nilotinib (a newer treatment in this class) was not as effective as high-dose imatinib as first-line therapy in terms of PFS and overall survival (OS) over 2 years.13 More recently, in phase III clinical trials, the TKIs sunitinib and regorafenib have demonstrated efficacy in advanced GIST after failure of imatinib or other treatments.14,15 At present, the median PFS in GIST is approximately 3 years and new treatments are needed to improve this.
The kinase inhibitory profiles of imatinib, sunitinib and regorafenib are different and, as a result, it is not surprising that their clinical performance in GIST also differs.16–18 Heterogenicity of primary and secondary mutations in the kinase enzyme set (kinome), principally in exons 13, 14, 17 and 18, result in variable resistance profiles to TKIs and in problems treating GIST with either imatinib or sunitinib.19 Despite encouraging results, it should be stressed that there is no evidence that regorafenib has better clinical efficacy in GIST than imatinib or sunitib and no headto- head studies have been conducted. These medications are beneficial in different situations and there can be advantages in switching between them as discussed below.
The multikinase-inhibiting mechanism of action of regorafenib can provide extended efficacy and control of GIST even in the presence of some secondary mutations. This was demonstrated in the long-term follow-up of a phase II study (n=33) in patients with metastatic GIST who were treated with regorafenib (160 mg OD).20 The median follow-up was 41 months, and only four patients (12%) had no disease progression. Overall PFS was 13.2 months and OS was 25.0 months. However, the longest median PFS (13.4 months) was seen in patients with a primary KIT exon 11 mutation, whereas patients with KIT/PDGFRA wild-type, non-SDH-deficient tumours showed a much shorter median PFS (1.6 months, p<0.0001).
Further evidence of regorafenib efficacy in GIST was provided by the multicentre phase III GIST Regorafenib In Progressive Disease (GRID) trial.
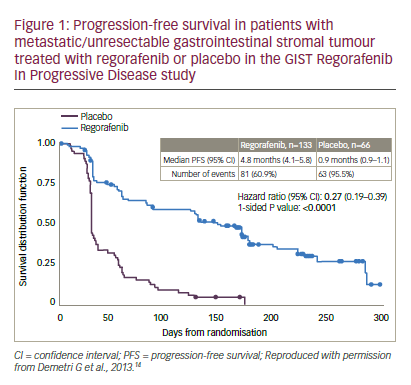
14 Patients with metastatic or unresectable GIST, who had failed on at least imatinib and sunitinib were randomised to receive either regorafenib (n=133) or placebo (n=66), with both groups receiving best supportive care (BSC). When disease progression occurred, patients were unblinded; those receiving placebo were crossed over to regorafenib or continued on active treatment until the next progression. The patient populations in the treatment and placebo groups were balanced in terms of gender, race, proportions who received two or more prior lines of GIST therapies and Eastern Cooperative Oncology Group (ECOG) status (0 or 1). The median PFS for regorafenib was 4.8 months versus 0.9 months for placebo (hazard ratio [HR]: 0.27 p<0.0001, see Figure 1). Due to progression, 56/66 (85%) placebo-treated patients switched to regorafenib, which resulted in there being no significant difference in OS (HR: 0.77, p=0.199). This lack of difference was a result of the study design and was expected.
Pre-specified analyses of the GRID study findings showed benefits of regorafenib in all subgroups: geographical region (north America, Asia, rest of world), gender, race, age (< or >65 years), body mass index (BMI), ECOG score (0 or 1), duration of previous imatinib treatment (<6, ≥6–<18 and ≥18 months) or use as third- or fourth-line treatment (HR 0.15–0.50).14 A subsequent exploratory analysis showed that 56.7% of regorafenib and 47.2% of placebo-treated patients had KIT exon 11 mutations. For these patients, PFS durations were: 5.6 and 1.1 months, respectively. In addition, 15.0% and 16.7% had KIT exon 9 mutations, respectively. For these, PFS durations were: 5.4 and 0.9 months, respectively. This shows that regorafenib was effective for both mutation types. Other mutations (for example, PDGFRA) were present in too few patients to derive any meaningful conclusions. The main treatment-emergent adverse events for regorafenib versus placebo were hand-foot skin reaction (20% versus 0%), hypertension (23% versus 3%) and diarrhoea (5% versus 0%). Based on the GRID study findings, regorafenib was approved for use in GIST in both the US and Europe.
The importance of investigating the genotype of GIST patients was further emphasised by a mutational analysis of baseline plasma (n=163) using BEAMing techniques and tumour tissue analysis (n=102) using Sanger sequencing on samples from the GRID study21 (BEAMing assays [Beads, Emulsions, Amplification, Magnetics] involve a highly accurate and sensitive form of digital polymerase chain reaction that is designed
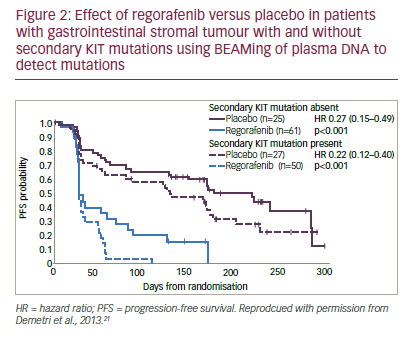
to detect specific commonly observed mutations in ‘hot spot’ locations using blood samples).22 Preliminary findings, as yet published in abstract form only indicate that these techniques found any KIT mutations in 58% and 66% of plasma and tumour samples, respectively. Exon 9 mutations were found in 15% and 18%, exon 11 mutations in 12% and 43% and secondary KIT mutations in 47% and 12%, respectively. KIT mutational status appeared to be correlated with imatinib and sunitinib treatment duration. Subgroup analysis based on mutational status showed an improved PFS in regorafenib-treated patients versus placebo-treated patients in all subgroups by both central and local review of imaging studies. In addition, secondary KIT mutations appeared to be associated with poorer PFS prognosis (see Figure 2) but the efficacy of regorafenib versus placebo did not appear to be affected by the type of KIT mutations present. These results suggested that plasma analysis could be used to screen mutations in GIST.

The case example given above presents one patient’s experience of GIST and the eventual failure of imatinib and then sunitinib. This case is interesting in that, having started regorafenib in response to disease progression, after approximately 5 months he showed further progression (peritoneal lesions) but the therapy was continued. After a further 10 months, a response was seen while he was still receiving regorafenib and long-term PFS has been maintained. While this is an experience from a single patient, it could indicate that
some apparent metastatic progression may not always represent real disease progression and may not justify an immediate change of therapy. In some circumstances, maintaining therapeutic pressure can be important. The ability of regorafenib to control the disease for an extended period of time is encouraging and suggests that it is an effective therapeutic option for advanced GIST where first- to third-line treatments have failed.
The Rechallenge of Imatinib in GIST Having no effective Treatment (RIGHT) study investigated the potential of reusing therapies in GIST after earlier failure.23 Patients with GIST (n=81) who had shown prior clinical benefit from first-line imatinib but had progressed on that treatment and on subsequent sunitinib and possibly a third-line TKI were randomised to imatinib or placebo. On further disease progression, placebo patients could be crossed over to imatinib and those receiving imatinib could be stopped or continued. Imatinib was well tolerated; PFS was significantly longer for imatinib arm compared with placebo, (1.8 months versus 0.9 months, p=0.00075) and a disease control rate at 12 weeks of 32%. There were no significant improvements in OS for imatinib versus placebo due to the study being a cross-over design.
A different approach to treating GIST is being taken by an ongoing randomised phase II trial of imatinib alternating with regorafenib compared with imatinib alone for the first-line treatment of advanced GIST (NCT 02365441).24 Patients (n=240) who have received no other treatment for metastatic disease are randomised to either continuous imatinib or to a regimen of imatinib for 21–25 days, a three- to seven-day gap, then regorafenib for 21 days followed by a seven-day gap, and then continuing repeats of this cycle. The primary outcome measure is PFS at 24 months; other endpoints include objective tumour response rate, OS and adverse events. Follow-up will continue for 5 years and the study is due to complete in 2020. This is an important study that has the potential to identify a new and effective approach to first-line therapy in advanced GIST.
Soft tissue and visceral sarcoma
The TKIs have also shown efficacy in the treatment of STS and visceral sarcomas after other treatments have failed. This was shown in a trial of patients (n=369) with angiogenesis-inhibitor-naïve, metastatic STS that were progressing despite chemotherapy who were randomised 2:1 to receive pazopanib 800 mg OD or placebo.25 The median PFS was 4.6 months versus 1.6 months for pazopanib and placebo (HR: 0.31, p<0.0001) and the proportions with PFS at 6 months were 37.7% and 7.0%, respectively. The most common adverse events were fatigue, diarrhoea, nausea, weight loss and hypertension.
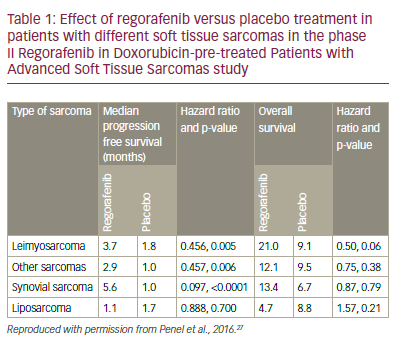
These encouraging results led to the phase II Regorafenib in doxorubicin-pre-treated patients with advanced soft tissue sarcomas (REGO-SARC-1214) study.26,27 Patients with advanced STS were stratified into four groups, (liposarcoma [n=50], leimyosarcoma [LMS, n=50], synovial sarcomas [n=25] or other sarcomas [n=50]) and randomised to regorafenib (160 mg OD for 3 weeks [3 weeks on, 1 week off] or placebo (both with BSC). For leiomyosarcomas, synovial sarcomas and other sarcomas there were significant increases in median PFS for regorafenib compared with placebo (p=0.005, 0.006, and <0.0001, respectively, see Table 1). For liposarcomas, however, there was no apparent difference in PFS between regorafenib and placebo treatment. There were also numerical (non-significant) improvements in OS for leiomyosarcomas, synovial sarcomas and other sarcomas but not for liposarcomas. The observed toxicities of regorafenib in this study were consistent with the known safety profile; most common adverse events being asthenia (63%), diarrhoea (44%), mucositis (44%), hand-foot skin syndrome (44%), anorexia (38%) and arterial hypertension (36%). The pooled analysis (see Figure 3) shows a significant advantage for regorafenib over placebo for PFS (HR: 0.36, p<0.0001) and a trending significant improvement for OS (HR: 0.67, p=0.06). These results represent an advance in the treatment of STS and the results of ongoing studies of patients with STS who were pre-treated with pazopanib will provide further insights.
A substantial unmet clinical need is effective therapy for BS. After firstline treatments, there is a shortage of further therapies. The potential of regorafenib in treating BS is currently being investigated in the phase II REGOBONE study (planned n=132, NCT02389244). In this trial, patients with conventional high-grade osteosarcoma, Ewing sarcoma of bone or chondrosarcoma (localised, unresectable cases can be included) are randomised to regorafenib 160 mg OD for 3 weeks or placebo (both with BSC). The treatment will continue until there is progression (as determined by Response Evaluation Criteria In Solid Tumors [RECIST] review) when treatment will be unblinded and continued if justified and placebo patients can be switched to regorafenib. Completion of the study is expected in 2020.
In summary:
• regorafenib has proven efficacy in GIST, shows potential in STS and is being evaluated in BS;
• analysis of mutations in kinase genes in GIST can inform treatment in advanced disease;
• selecting suitable patients and continued and monitoring enables longer treatment duration and potentially longer PFS;
• tumour heterogeneity and resistance are serious problems and limit successful treatment; and
• novel concepts are needed in the development of new approaches to treating GIST, STS and BS.
The emerging role of multikinase inhibitors for treatment of refractory advanced oesophagograstric cancer
Gastric and oeosphageal cancers are, respectively, the third and sixth most common causes of cancer-related deaths worldwide.28 Oesophagogastric cancer is, therefore, a serious and substantial clinical problem for oncologists and has very high fatality rates.29 Treatment options are limited, with the only potentially curative option being resection. The limited survival rates result from the majority of patients presenting with advanced disease or developing metastases after receiving treatment
with curative intent.30 Effective treatments in oesophagogastric cancer, therefore, are an urgent and largely unmet medical need.
Current European Society for Medical Oncology/European Society of Surgical Oncology/European Society for Radiotherapy and Oncology (ESMO/ESSO/ESTRO) clinical practice guidelines and National Comprehensive Cancer Network (NCCN) guidelines indicate that in cases of inoperable metastatic or locally advanced oesophagogastric junction adenocarcinoma, patients should receive only BSC if they are unfit for treatment.31,32 Those who are fit to receive treatment can be given palliative chemotherapy; HER2-positive patients can receive trastuzumab with a platinum-based compound. HER2-negative patients are suitable for fluoropyrimidine and a platinum treatment or a triplet combination (for example, epirubicin, oxaliplatin and capecitabine [EOX] or docetaxel, cisplatin and 5-fluorouracil [DCF]). Alternatively, novel agents accessed in clinical trials could be considered. After treatment failure with these first-line therapies, second-line approaches for metastatic or locally advanced oesophagogastric junction adenocarcinoma include: ramucirumab with paclitaxel, docetaxel, paclitaxel, irinotecan-based regimens or ramucirumab (the only approved agent for use in advanced gastric cancer).31,32 The impact of these second-line treatments, however, remains modest. The most frequent choice of therapy for refractory oesophagogastric cancers among attendees at this symposium was single-agent chemotherapy with or without ramucirumab.
Despite this gloomy situation, some more effective treatments may be emerging. Following the successful use and approval of regorafenib in metastatic colorectal cancer and GIST,33,34 the drug also showed promising results in oesophagogastric cancer in the phase II Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE) study.35,36 Patients (n=152) with metastatic or locally recurrent oesophagogastric junction or stomach, adenocarcinoma or undifferentiated histology (not oesophageal cancer) were randomised 2:1 to receive regorafenib 160 mg or placebo (both with BSC) on days 1–21 of successive 28-day cycles and were treated until disease progression or prohibitive adverse events occurred. The median duration of the regorafenib therapy was 8 weeks, the median dose intensity was 150 mg/day (130 mg/day in Korea and 160 mg/day in Australia, New Zealand and Canada); 80% of patients reached a dose of 160 mg/day. Following progression, 29/50 patients in the placebo group switched to regorafenib with a median time on active treatment of 7 weeks.
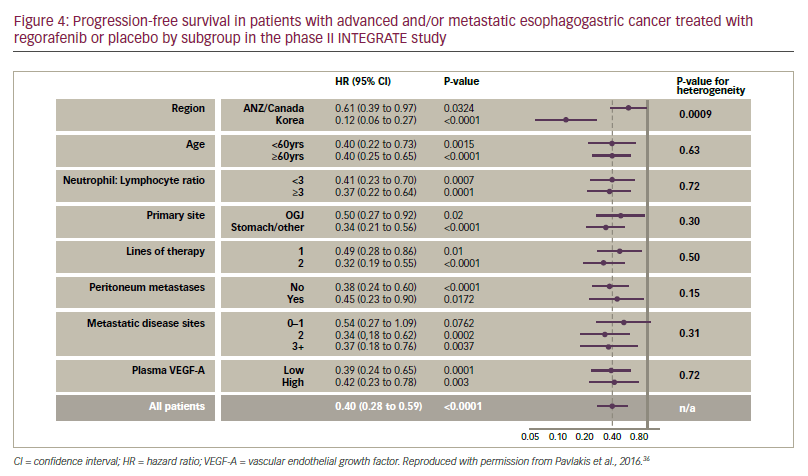
The median PFS in the INTEGRATE study (primary endpoint) was significantly longer with regorafenib compared with placebo (2.6 months versus 0.9 months [HR. 0.40, p<0.0001]). Almost all subgroup analyses (age, primary site, neutrophil ratio, lines of therapy and plasma vascular endothelial growth factor-A level) showed significantly longer PFS for regorafenib, although there was a regional effect; treatment of patients in Korea had a more pronounced effect on PFS versus placebo than for those in Canada, Australia or New Zealand (see Figure 4). This regional inconsistency may been due to differences in screening programmes, leading to larger proportions of patients with more advanced disease entering the study and affecting PFS in some territories. For regorafenib versus placebo, stable disease was achieved in 40% versus 14% and clinical benefit at 2 months was reported in 45% versus 18%, respectively. In addition, there was a numerical improvement in OS with regorafenib versus placebo (median OS 5.8 months versus 4.5 months, HR 0.74, p=0.11). The toxicity profiles were similar to that previously reported; the most frequent treatmentassociated adverse events (>20%) were fatigue, anorexia, hypertension, constipation and nausea which were mostly Grade 1-2 in severity.
The apparent efficacy of regorafenib in the INTEGRATE trail has prompted the establishment of the phase III INTEGRATE II trail.37 In this planned study, patients with metastatic or locally recurrent oesophagogastric cancer (n=350, with adenocarcinoma or undifferentiated carcinoma who have failed or are intolerant to two prior lines of anti-cancer therapy) will be randomised to regorafenib 160 mg once daily (3 weeks on/1 week off) or placebo (both with BSC) until disease progression. The primary endpoint will be OS; secondary endpoints will include PFS, response rate, quality of life, safety, biomarkers and pharmacokinetics. The study is scheduled to complete in 2019 and the results will more clearly indicate whether regorafenib has any efficacy in oesophagogastric cancer.
In summary: • treatment options, particularly for second-line use, for inoperable or locally advanced oesophagogastric cancers are currently limited and patient prognosis is bleak;
• the phase II INTEGRATE study showed that regorafenib significantly increased PFS in oesophagogastric cancer versus placebo in various different patient populations and subgroups;
–– the greatest effect on PFS was seen in Korean patients but significant effects were seen in all geographic locations;
–– regorafenib was generally well tolerated and the observed toxicities were consistent with those previously reported and most were Grades 1-2; and
• the promising results of the INTEGRATE study justified the establishment of the planned phase III INTEGRATE II study, which will evaluate regorafenib in advanced/metastatic oesophagogastric cancer in a larger group of patients.
Conclusion
Clinical trial evidence indicates that targeting multiple different TK mutations in tumour cells provides increased efficacy in GIST. Experience with TKIs shows that they are effective in GIST after other treatments have failed and patients can be switched between them. Given the heterogenicity of TK mutations, it is vital that patients undergo genetic profiling to indicate treatments that are most likely to be effective. Regorafenib is a fairly new addition to the set of treatments that are approved for use in GIST and its notable efficacy appears to be a result of its action against greater numbers of TK mutation types (for example, KIT exon 11 mutations) compared with other TKIs. Regorafenib also shows a manageable safety profile when used in this indication. Animal models show that regorafenib targets both tumour cells and abundant stromal cells around them38 and effectively contains the tumour, although investigation of this action in human cells is needed.
The case example of a 67-year-old patient with GIST shows that, in some patients, with suitable tumour genetic profiles, TKI treatments can provide PFS for extended durations. The PFS of 50 months on imatinib and 63 months with regorafenib are impressive and this patient has survived over 12 years since first diagnosis. It is notable that, despite progression of peritoneal lesions, the regorafenib treatment was maintained and the patient subsequently benefitted. This raises important questions about the nature of progression in GIST and other cancers and when a change of therapy is justified. This case also indicates that as GIST progresses, it may be more important to regard OS as the most important outcome rather than PFS.
In GIST, just as in metastatic colorectal cancer, therapeutic benefit can also be gained from re-challenging patients with a TKI they have previously progressed on. This has the potential to increase the therapeutic options available in advanced disease; the RIGHT study showed significant gains in PFS using this strategy although there was no overall gain in OS.39 Further exploration of this approach, however, may reveal greater efficacy. The phase II study alternating imatinib and regorafenib as first-line therapy in GIST over 5 years is a novel approach and may also provide increased efficacy and extend survival.24
The results of studies investigating regorafenib in the treatment of STS are encouraging and suggest a potential new treatment option for different types of STS other than liposarcomas. In addition, the phase II evaluation of regorafenib in BS is of considerable interest since treatment options are seriously lacking and the outlook for patients with this condition are currently poor. The results of this study are awaited with interest.
A further potential indication for regorafenib is oesophagogastric cancer. These cancers are common and have very high mortality rates with few effective treatments available. The promising improvements seen in PFS in almost all subgroups in the INTEGRATE trial suggest that it is possible to extend survival in advanced disease. It remains to be seen whether these positive findings are confirmed or even improved in the larger INTEGRATE II study.
In GIST, STS, BS and oesophagogastric cancers there remains an urgent need for more effective treatments. The results outlined above, however, indicate that the existing TKIs, in particular regorafenib, are valuable treatments in these multiple different cancer types and their potential indications are expanding. Greater use of these drugs, development of optimal regimens for their use/sequencing and improved ability to identify the most suitable patients to receive them may improve prognosis and survival in these difficult to treat cancers in the near future.













