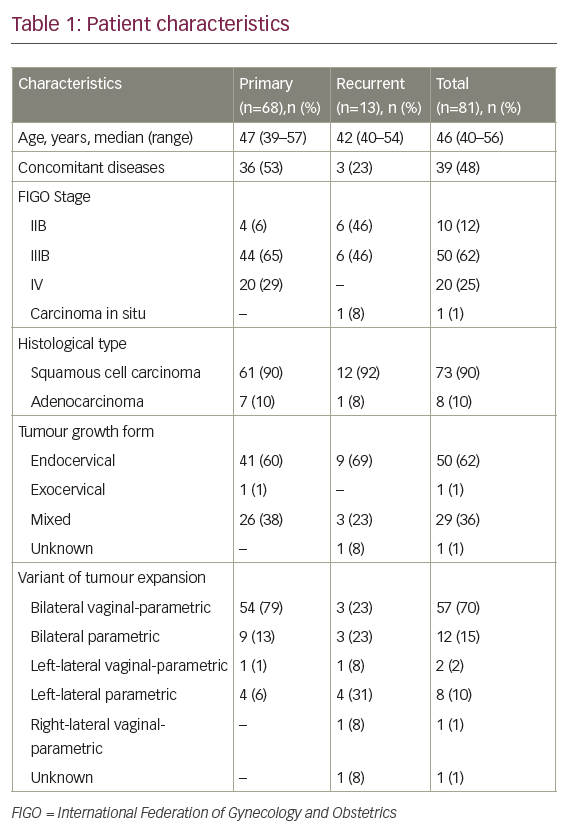
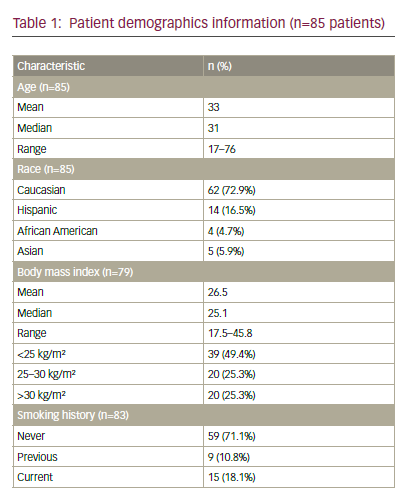
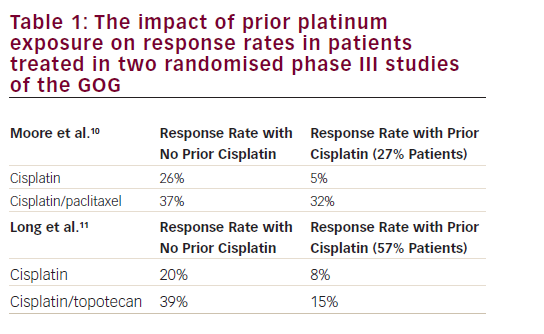
Factors that predict outcome in relapsing patients include previous complete response (CR), non-mediastinal primary, low serum tumour markers and no visceral metastasis. High-dose chemotherapy (HDCT) plus peripheral blood stem cell transplantation (PBST) can help patients who relapse after conventional chemotherapy, but has not been shown to be effacious.3 A multi-institutional retrospective study of 310 patients treated with HDCT and PBST identified prognostic factors of response to HDCT and failure-free survival (FFS) in patients with relapsed or cisplatin-refractory germ cell tumours.4 This article reviews new or targeted agents in relapsed or cisplatin-refractory germ cell tumours. Cisplatin-refractory disease is defined as the partial response of the disease during cisplatin-based chemotherapy, with subsequent progression within four weeks of treatment. Absolute cisplatin-refractory disease is defined as tumour progression during cisplatin-based chemotherapy.
New Drugs Paclitaxel
Paclitaxel, a member of the taxoid family, is an antimicrotubule agent that promotes the assembly and stabilisation of microtubules from tubulin dimers. The drug has been investigated in three phase II trials.5–7 All patients were heavily pre-treated with standard chemotherapy regimens. Many had cisplatin-refractory disease and received a third or subsequent line of treatment. At a dose of 2256 to 250mg/m2,5 paclitaxel induced an objective response in 12 of 51 patients (23%). A response occurred in only two of 18 patients (11%) with doses of between 170 and 200mg/m2.7 These studies provided interesting results and sufficient background to include paclitaxel in both first-line8 and salvage treatments.9
Paclitaxel, Ifosfamide and Cisplatin First-line Salvage Therapy
Paclitaxel-based combinations have been tested for standard salvage chemotherapy using a paclitaxel plus ifosfamide and cisplatin (TIP) regimen9 and in combination with sequential HDCT.10 At the Memorial Sloan Kettering Cancer Center (MSKCC), paclitaxel was given at a dose of 200mg/m2 administered as a 24-hour continuous infusion on day one of the cycle. In the German group, paclitaxel was given on day one at a dose of 175mg/m2 and was administered as a three-hour infusion.11 The same dose and schedule were used in trials published by the Medical Research Council (MRC)12 and Mardiak et al.13 In the trial conducted by the French group, the dose of paclitaxel was 250mg/m2 administered as a three-hour infusion.14 At the MSKCC, the TIP regimen9 was administered as first-line salvage therapy in favourable-risk patients. Thirty patients were treated: the rates of CR, continuous CR and non-evolutive disease (NED) were 80, 73 and 80%, respectively. Similar TIP protocols induced a CR rate of 60% in 43 patients included in the MRC trial,12 and 65% in the study by Mardiak et al.13
Combination of Paclitaxel and High-dose Chemotherapy plus Peripheral Blood Stem Cell Transplantation in First-line Salvage Therapy
The German group developed a protocol with three cycles of TIP followed by one cycle of high-dose etoposide, carboplatin and thiotepa.11 Eighty patients were treated: 67% were treated in the first-line salvage setting, 76% were cisplatin-sensitive and only 35% had achieved previous CR. One patient experienced a toxic death and 18 patients did not receive HDCT. The CR, continuous CR and NED rates were 35, 26 and 33%, respectively.
The French group has developed a protocol with two cycles of epirubicin plus paclitaxel followed by one cycle of high-dose cyclophosphamide and thiotepa and two cycles of high-dose carboplatin plus etoposide (CE).14 Forty-five patients were studied. Of these, 15% were in the first-line salvage setting (refractory disease). Only 33 patients received HDCT, of whom 22 completed the programme. There were five treatment-related deaths. The CR, continuous CR and NED rates were 22, 19 and 23%, respectively. The MSKCC group also developed a sequential dose-intensive protocol of chemotherapy known as TICE (two cycles of paclitaxel plus ifosfamide followed by three cycles of high-dose CE).10 Thirty-seven patients were included in the trial: all had unfavourable characteristics (84% of the patients were intermediate-risk and had extragonadal primary and more than one line of treatment). There were no toxicity-related deaths. The CR, continuous CR and NED rates were 62, 41 and 49%, respectively. Neither low-15 nor high-dose16 paclitaxel in the HDCT regimen was superior.
Table 1: Results of New Drugs in Salvage Treatment
| Authors (references) | Regimen | No. Pts | No. EG | No. RD (%) | No. ARD (%) | Prior HDCT No. (%) | Late Relapse | CR (%) | PR- (%) | PR+ (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Tumours (%) | ||||||||||
| Motzer5 | Paclitaxel | 31 | 11 (33) | 0 | 0 | 5 (16) | ND | 3 (10) | 8 (26) | ND |
| Bokemeyer6 | Paclitaxel | 24 | 3(12) | 18 (75) | 0 | 12 (50) | 0 | 2 (8) | 4 (17) | 0 |
| Sandler7 | Paclitaxel | 18 | 5 (28) | 18 (100) | 0 | 3 (17) | 6 (33) | 1 (5) | 1 (5) | 1 (5) |
| Bokemeyer18 | Gemcitabine | 35 | 8 (23) | 17 (49) | 17 (49) | 22 (63) | 4 (11) | 0 | 2 (6) | 4 (11) |
| Einhorn19 | Gemcitabine | 21 | 5 (24) | 13 (62) | 0 | 11 (52) | 2 (10) | 1 (5) | 2 (10) | 2 (10) |
| Kollmansbeger21 | Oxaliplatin | 32 | 5 (16) | 20 (62) | 7 (22) | 25 (78) | 11 (34) | 0 | 1 (3) | 3 (9) |
| Kollmansberger24 | Irinotecan | 15 | 3 (20) | 7 (47) | 4 (27) | 13 (87) | 2 (13) | 0 | 0 | 0 |
| Miki25 | I/CDDP or I/NDP | 11 7 | }2 (10) | }20 (100) | }0 | }4 (20) | }ND | 1 (9) 1 (14) | 4 (36) 2 (28) | 0 1 (14) |
| Pectasides32 | I/Oxa | 18 | ND | 18 (100) | 0 | 0 | 1 (5) | 4 (22) | 3 (17) | 3 (17) |
| Hinton28 | Gem/Pacl | 28 | 7 (25) | 10 (36) | 0 | 10 (36) | ND | 3 (11) | 3 (11) | 3 (11) |
| Einhorn (II)26 | Gem/Pacl | 32 | 0 | ND | ND | 32 (100) | ND | 6 (19) | 4 (12) | 4 (12) |
| Theodore33 | Oxal/Pacl | 27 | 7 (4) | 16 (59) | 0 | 5 (18) | 0 | 0 | 0 | 1 (3) |
| Kollmansberger30 | Gem/Oxa | 35 | 5 (14) | 13 (37) | 9 (26) | 31 (89) | 10 (29) | 3 (9) | 2 (6) | 11 (31) |
| Pectasides29 | Gem/Oxa | 29 | 2 (7) | 24 (83) | 5 (17) | 4 (14) | 2 (7) | 4 (14) | 5 (17) | 5 (17) |
| De Giorgi31 | Gem/Oxa | 18 | 6 (33) | 7 (39) | 11 (61) | 4 (22) | 2 (11) | 1 (6) | 1(6) | 1(6) |
| Shamash34 | I/Oxa/Pacl | 28 | 5 (18) | 3 (11) | 5 (18) | 0 | ND | 5 (18) | 13 (46) | 2 (7) |
| Bokemeyer36 | Gem/0xa/Pacl | 41 | 9 (22) | 9 (22) | 5 (12) | 32 (78) | 3 (7) | 2 (5) | 14 (34) | 5 (12) |
| De Giorgi35 | Gem/0xa/Pacl | 9 | ND | 9 (100) | 0 | ND | ND | 0 | 1 (5) | 1 (5) |
First-line Treatment
De Wit et al. treated 14 patients with intermediate- and poor-prognosis germ cell tumours in a phase I/II study of paclitaxel plus BEP as first-line chemotherapy.8 All of the 13 evaluable patients obtained a complete response. The recommended dose of paclitaxel was 175mg/m2. A randomised phase II/III study of paclitaxel, bleomycin, etoposid and cisplatin (T-BEP) versus BEP is ongoing within the European Organisation for Research and Treatment of Cancer (EORTC) for patients with an intermediate prognosis according to the International Germ Cell Classification Consensus Group (IGCCCG). The German Testicular Cancer Group has evaluated the feasibility and toxicity of sequential dose-intensified chemotherapy combined with paclitaxel plus PBSC for patients with poor-prognosis untreated metastatic germ cell tumours.17 Paclitaxel was combined with high-dose etoposide, ifosfamide and cisplatin at three dose levels (135, 175 and 225mg/m2) on day six. Forty-one of 53 patients (79%) achieved a favourable response to chemotherapy plus surgery. Myelosuppression was the major adverse effect. After a median follow-up of 41 months, the five-year overall survival rate was 75.2% (95% confidence interval [CI] 62.5–87.8%). The French group is currently conducting a randomised trial in poor-prognosis disease comparing standard BEP with dose-dense chemotherapy (including oxaliplatin, paclitaxel, ifosfamide and the BEP regimen) in patients with little decrease in serum tumour marker levels.
Gemcitabine
2’deoxy-2’2’-difluorocytidine monohydrochloride (gemcitabine) is a pyrimidine analogue. Its mechanism of action depends on phosphorylation and inhibition of the production of deoxynucleotide triphosphate, required for DNA synthesis. Two phase II trials conducted by the German Testicular Study Group and the Indiana University have confirmed the efficacy of gemcitabine as a single agent in relapsed or cisplatin-refractory disease, with response rates of 19 and 15%, respectively.18,19 At Indiana University, gemcitabine was administered at a dose of 1,200mg/m2/day on days one, eight and 15 every four weeks. Thirty per cent of the patients had grade 3 leucopenia. None received platelet transfusion. In the German study, patients received gemcitabine 1,000mg/m2/day on days one, eight and 15 every four weeks. Haematological toxicity was more severe in this study, with grade 3/4 neutropenia in 13% of patients and thrombocytopenia in 20%. Dose reduction was mandatory in 32% of patients.
Oxaliplatin
Oxaliplatin is a water-soluble derivate of 1,2diaminocyclohexane platinum that is non-cross-resistant to cisplatin.20 Oxaliplatin has demonstrated in vitro activity in cisplatin-resistant non-seminomatous germ cell lines; this is why it was tested for the treatment of cisplatin-refractory germ cell cancer patients. The German Testicular Cancer Study Group has performed a phase II trial with oxaliplatin 130mg/m2/day on days one and 15 or 60mg/m2/day on days one, eight and 15 as a single agent in refractory germ cell tumours.21 Four of 32 treated patients achieved a partial response. The treatment was well tolerated.
A French group tested oxaliplatin 130mg/m2 on day one plus cisplatin 100mg/m2 on day one every three to four weeks in 13 patients who relapsed after first-line cisplatin-based chemotherapy.22 Seven patients showed a response. Of the patients, 13% developed grade 3 thrombocytopenia and one developed grade 4 thrombocytopenia. No fever or treatment-related deaths ocurred. In good-risk relapsed patients, the French group is conducting a trial testing the association of gemcitabine 1,000mg/m2 on days one and five, ifosfamide 1,200mg/m2 on days one to five and cisplatin 20mg/m2 on days one to five every 21 days (GIP-TG).Irinotecan
Irinotecan is a topoisomerase I inhibitor. The rationale for using irinotecan in refractory testicular germ cell tumours is based on the results published by Miki et al., who showed the antitumour activity of irinotecan as a single agent or in combination with cisplatin on human germ cell tumours xenografted to nude mice.23
| Authors (reference) | Drug | No. Patients | RD or ARD | CR | PR | PR+ | SD |
|---|---|---|---|---|---|---|---|
| Beer et al.62 | Arsenic trioxide | 20 | 9 | 0 | 0 | 0 | 0 |
| Moasser et al.59 | Tretinoin | 16 | 16 | 0 | 0 | 0 | 0 |
| Gold et al.60 | Isotretinoin | 15 | 15 | 0 | 0 | 0 | 4 |
| Rick et al.61 | Thalidomide | 15 | 15 | 0 | 0 | 5 | 0 |
| Motzer et al.58 | Suramin | 14 | 14 | 0 | 0 | 1 | 0 |
| Einhorn et al.70 | Imatinib | 6 | 6 | 0 | 0 | 0 | 0 |
| Pedersini71 | Imatinib | 1 | 1 | 1 | 0 | 0 | 0 |
| Kollmansberger75 | Trastuzumab | 1 | 0 | 0 | 0 | 1 | 0 |
| Mego et al.78 | Bevacizumab | 1 | 1 | 0 | 0 | 0 | 1 |
| Voigt et al.77 | HDCT plus bevacizumab | 1 | 1 | 0 | 0 | 1 | 0 |
No. = number; HDCT = high-dose chemotherapy; RD = refractory disease; ARD = absolute refractory disease; CR = complete response; PR- = partial response marker normalised; PR+ = partial response marker elevated; SD = stable disease.
The German Testicular Cancer Study Group performed a phase II study with irinotecan 300–350mg/m2 every three weeks as a single agent in patients with relapsed or cisplatin-refractory germ cell tumours.24 Toxicity was acceptable, but the trial failed to demonstrate any activity of the treatment as no response was observed among the 15 treated patients.Miki et al. investigated the efficacy of irinotecan 100–150mg/m2/day on days one and 15 or 200–300mg/m2/day on day one plus cisplatin 20mg/m2/day on days one to five or nedaplatin 100mg/m2/day on day one every four weeks in refractory germ cell tumour patients.25 Twenty patients were enrolled in the study; the response rate was 50% (two complete and seven partial responses). Myelosuppression was the major toxicity, but adverse effects were manageable.
Gemcitabine and Paclitaxel
Einhorn et al. published a retrospective review of their experience with gemcitabine plus paclitaxel.26 The rationale for combining the two drugs was based on the results of phase I and II trials.27,28 Thirty-two patients with relapse after salvage treatment with HDCT and PBST were subsequently treated with paclitaxel 100mg/m2/day and gemcitabine 1,000mg/m2/day on days one, eight and 15 every four weeks. Ten of 32 patients (31%) achieved objective response (six complete responses and four partial remissions). The main adverse events were neurological and haematological toxicities. This regimen was shown to offer long-term response.
Oxaliplatin and Gemcitabine
The activity of the two drugs as single agents has been demonstrated in refractory patients and their combination has been shown to be supra-additive in in vitro models. Based on these results, Pectasides et al.29 and Kollmannsberger et al.30 tested the combination in heavily pre-treated patients in two phase II trials following the same schedule of gemcitabine 1,000mg/m2/day on days one and eight and oxaliplatin 130mg/m2/day on day one every three weeks. Pectasides et al.29 treated 29 cisplatin-refractory patients, nine of whom (32%) achieved an objective response. Kollmannsberger’s study30 enrolled 35 patients, 16 of whom (46%) achieved objective responses. The non-haematological side effects were grade 3 neurotoxicity in three patients. Seventeen (48%) and 19 (55%) patients developed grade 3/4 thrombocytopenia and leukocytopenia, respectively. One of them (3%) died as a result of therapy-related sepsis. De Giorgo et al.31 developed a slightly different schedule: gemcitabine 1,250mg/m2/day on days one and eight and oxaliplatin 130mg/m2/day on day one every three weeks. Three of the 18 patients enrolled achieved an objective response. The combination of gemcitabine and oxaliplatin has demonstrated antitumour activity in heavily pre-treated patients with cisplatin-refractory germ cell cancer.
Oxaliplatin and Irinotecan
Pectasides et al.32 treated 18 cisplatin-refractory germ cell tumour patients in the third-line setting using a combination of irinotecan 80mg/m2/day on days one, eight and 15 and oxaliplatin 85mg/m2/day on days one and 15. Seven patients (40%) achieved an objective response. The most common toxicities were gastrointestinal: grade 3/4 diarrhoea and nausea/vomiting in 22 and 28% of patients, respectively. Oxaliplatin and Paclitaxel
A French group performed a phase II trial in 27 patients with refractory disease or early relapse after cisplatin-based chemotherapy.33 Patients received paclitaxel 175mg/m2 and oxaliplatin 130mg/m2 on the same day every three weeks. Results were disappointing because only one patient responded to the treatment. The main toxicity was haematological. The low dose of paclitaxel (<225mg/m2) may account for this response rate.
Oxaliplatin, Irinotecan and Paclitaxel
The rationale for the combination of these three drugs is the activity of the doublets or of each drug used as a single agent in refractory germ cell tumours. Twenty-eight patients were enrolled in a phase II trial.34 All had received at least two cisplatin-based chemotherapy regimens. The treatment schedule was as follows: irinotecan 200mg/m2/day on day one, paclitaxel 80mg/m2/day on days one, eight and 15 and oxaliplatin 200mg/m2/day on day one. Eighteen patients (64%) obtained an objective response (five CR, 13 PR-). This regimen was considered toxic because 68% of the patients showed signs of infection.
Oxaliplatin, Gemcitabine and Paclitaxel
The first trial with the three most active drugs, oxaliplatin plus gemcitabine and paclitaxel, has been published by De Giorgio et al. in nine cisplatin-refractory germ cell tumour patients.35 The chemotherapy regimen had to be modified because of excessive haematotoxicity, and the authors concluded that it was not feasible in this patient group. The German Testicular Cancer Study Group published a trial of the combination of the three drugs with the following schedule: gemcitabine 800mg/m2/day and paclitaxel 80mg/m2/day on days one and eight, and oxaliplatin 130mg/m2/day on day one every three weeks.36 Forty-one patients with cisplatin-refractory disease or with relapse after HDCT and PBST were treated, half of whom obtained an objective response. This combination was considered to be feasible and had acceptable toxicities. The main toxicities were haematological: grade 3/4 leucocytopenia in 15% of the patients, anaemia in 7% and thrombocytopenia in 49%.
New drugs such as paclitaxel, oxaliplatin and gemcitabine are active when used as single agents or in combination in heavily pre-treated patients. The response rates and numbers of long-term responders obtained with the association of paclitaxel, ifosfamide and cisplatin in good-risk patients receiving first-line salvage therapy are encouraging. However, there are too few of these patients to perform a phase III trial comparing VeIP with TIP regimens. The efficacy of paclitaxel and oxaliplatin in combination with the BEP regimen is being evaluated as first-line treatment in poor-risk groups.
New Targets
Intratubular germ-cell neoplasia unclassified (IGCNU) is the precursor of invasive testicular germ cell tumour.37 Patients with IGCNU develop invasive lesions within five years of diagnosis.38 Activating mutations of KIT39 and gain of material from 12p are warranted to the development of invasive tumour from IGCNU.40 All post-pubertal germ cell tumours are characterised genetically by having one or more copies of isochromosome 12p or excess 12p genetic material.41 Skotheim et al. have identified an amplified region at 12p11.2–12.1 in which several genes of interest (SOX5, JAW1 and K-RAS) are located.42 OCT3/4, NANOG and SOX2 are transcription factors that are implicated in embryonic development.43 44 Germ cell tumours show predominantly wild-type p53.4 Amplification, specific mutations and the overexpression of KIT and KRAS (which is one signalling pathway downstream from KIT) have been reported mainly in the treatment of seminoma.45–47 RAS and KIT activate a number of signalling molecules, including PI3-kinase (PI3K). PI3K is activated by a number of proteins, such as AKT3, which is generally overexpressed in the majority of non-seminoma and seminoma.48 PTEN, which is a tumour suppressor gene, inhibits PI3K activity. Di Vizio et al.49 reported that the loss of PTEN is implicated in the progression from ITGCNU to invasive tumours. A loss or decreased expression of PTEN is reported in 56 and 86% of seminoma and non-seminoma tumours, respectively.50,51 Godard et al. have shown evidence of a constitutive activation of the RAF/MEK/ERK pathway in germ cell tumours.52 Activated ERK (part of the MAPK signalling cascade) is found with a high level of expression in germ cell tumours.50,51 The gene GRB7, which that is located to 17q12, is overexpressed in germ cell tumours.53 GRB7 protein is a small molecule that binds directly to KIT and ERBB2,54,55 and may play an important role in the regulation of the downstream signalling of these different kinases. GRB7 is implicated in cell migration.56 KIT also activates members of the STAT family of proteins.57 STAT signalling is involved in migration and proliferation of the primordial germ cells.58 High levels of activated STAT3 are observed in most non-seminomas and seminomas.50,51
New targeted agents have been developed in refractory disease. Their study is based on both knowledge of molecular biology mechanisms involved in germ cell tumours and the empirical approach of new drugs. We present the data of targeted agents published in this setting.
Suramin
Suramin, a treatment of trypanosomes, also has antitumour activity. It blocks the activity of fibroblast growth factor and platelet-derived growth factor, and inhibits angiogenesis in vitro. Motzer et al. have reported a phase II trial of continious infusion of suramin in 14 cisplatin-refractory patients with germ cell tumours and failed to demonstrate an objective response.58
Retinoids
Retinoids induce cellular differentiation and inhibit growth in many solid tumours. All-trans retinoic acid, in combination with chemotherapy, is known to induce cellular differentiation in promyelocytic leukaemia. No objective response has been observed with all-trans retinoic acid in two phase II trials including germ cell tumour patients.59,60 Thalidomide
Thalidomide has antiangiogenic properties and cytotoxic activity. Fifteen patients with disease progression after two cisplatin-based chemotherapy courses have received thalidomide at doses escalated from 100 to a maximum of 600mg daily.61 No patient has achieved either complete or partial response. However, five of the 15 patients had decreased serum tumour marker levels, with a median response duration of three months.
Arsenic Trioxide
Arsenic trioxide has demonstrated activity in acute promyelocytic leukaemia. In the Southwest Oncology Group (SWOG), 20 heavily pre-treated germ cell tumour patients received arsenic trioxide at a dose of 0.25mg/kg/day administered intravenously over one to two hours on days one to five; the treatment was repeated every 28 days.62 There were no complete or partial responses. The median progression-free survival was one month and the median overall survival was two months. Severe toxicities were reported.
KIT (c-KIT Stem Cell Factor Receptor)
KIT is a 145kDa transmembrane glycoprotein, a member of the type III receptor tyrosine kinase family. Somatic mutations of the KIT gene have been reported in mast cell diseases and gastrointestinal stromal tumours. KIT is necessary for the migration and survival of primordial germ cells and is expressed in intratubular neoplastic germ cells and seminomas.63 Mutations of c-Kit in seminoma have been observed in exon 17 and, less frequently, in exon 11.64 In a retrospective study, Looijenga et al. have found a mutation of c-KIT on codon 816 in three of 224 (1.3%) unilateral and in 57 of 61 (93%) proven bilateral germ cell tumours.39 Bierman et al. have confirmed the risk of bilateral germ cell tumour when a somatic c-KIT mutation of codon 816 is present in the tumour.65 Unfortunately, these results have not been confirmed in recent publications.66 Coffey et al. analysed the percentage of activating mutations of c-KIT in exons 11 and 17 in a series of 220 germ cell tumours. Mutations were observed in all seminomas. In germ cell tumours, one activating mutation was found in exon 11 in a patient with unilateral disease, and eight in exon 17. One patient of 32 with bilateral germ cell tumours had an activating KIT mutation in exon 17 (3.1%).66
The most important retrospective analysis of KIT expression in germ cell tumours was published by Nikolaou.67 Thirty-eight of 49 patients (77.5%) with pure seminoma had positive staining for KIT. Scarce data are available in refractory germ cell tumours. In a series of 22 cisplatin-refractory germ cell tumours, only tissues from pure seminoma or with a seminoma component were positively stained for KIT.68 Madani et al.69 performed an immunohistochemical analysis of KIT expression in 23 tissue samples obtained from the most recently available metastatic site in cisplatin-refractory patients with non-seminomatous germ cell tumours. KIT was expressed in 11 of 23 tumours, especially in teratoma with malignant transformation and tissue samples from late relapses. A phase II study of imatinib has been performed at Indiana University in six refractory germ cell tumour patients who expressed KIT. No response has been observed.70 Pedersini et al. have reported a complete response to imatinib treatment in a patient with cisplatin-refractory seminoma who remained free of disease 24 months after the initiation of treatment.71
Epidermal Growth Factor Receptor
Epidermal growth factor receptor (EGFR) is a 170kDa transmembrane glycoprotein, a member of the type I receptor tyrosine kinase family. The protein is overexpressed in tumours of the neck, head, cervix and esophageous. EGFR expression has been analysed by histoimmunochemistry (HIC) in a large series of 182 germ cell tissue samples.72 EGFR staining was observed in 79% of teratomas with epithelial component, 29% of embryonal carcinomas and all syncytiotrophoblastic cells. No EGFR staining was observed in yolk sac tumours, seminomas and intratubular neoplasias. A smaller series published by Moroni et al.73 has reported the same pattern of EGFR staining according to histology. In relapsed patients, positive EGFR staining was observed in the majority of late relapses (more often yolk sac tumours) and in half of the patients with malignant transformation of teratoma.69 No clinical study with an EGFR-targeted agent has been published in germ cell tumours. Indiana University has just completed a trial with gefitinib; results are pending.HER-2/neu Protein
HER-2/neu is overexpressed in 25–30% of breast cancers and is associated with poor prognosis. The expression of the HER-2/neu protein and the amplification of the gene have been analysed in tissue samples of primary and refractory germ cell tumours. Ninety-six samples have been analysed using HIC for HER-2/neu protein expression and fluorescence in situ hybridisation (FISH) for HER-2/neu gene amplification.74 HER-2/neu protein was overexpressed in 22 of 96 samples of germ cell tumours, none of 22 primary testis tumours, eight of 25 active residual masses after chemotherapy, seven of 29 late relapses and seven of 20 primary mediastinal tumours. HER-2/neu gene amplification was observed in only four samples: three of 29 late relapses and one of 20 primary mediastinal germ cell tumours. The authors of the study concluded that the lack of correlation between HIC and FISH is not in favour of the development of clinical trials with trastuzumab. Kollmannsberger et al. reported a response to trastuzumab treatment in a patient with cisplatin-refractory disease.75
Anti-angiogenic Therapy
Vascular endothelial growth factor (VEGF), thymidine phosphorylase expression and vessel density have been evaluated using HIC in 80 germ cell tumours: 33 pure seminomas (25 stage I and eight metastatic) and 47 non-seminomatous germ cell tumours (20 stage I and 27 metastatic).76 VEGF protein was more often expressed in germ cell tissue than normal tissue, with 41% of germ cell tumour samples exhibiting positive staining for VEGF. The percentage of VEGF-positive cells correlated significantly with the microvessel count in germ cell tumours. The authors have shown that VEGF expression is associated with lymphovascular and venous involvement and the presence of metastases. Only two reports have been published on administration.77,78 The first patient had cisplatin-refractory disease and received high-dose chemotherapy (ifosfamide carboplatin and etoposide [ICE] regimen) in association with bevacizumab 7.5mg/kg on days one and 22.63 The patient achieved a partial response with more than 99% decrease of serum marker levels. Progression-free survival was five months. The second patient had inoperable growing teratoma and received bevacizumab 10mg/kg/day biweekly.78 The size of the tumour was stable for six months after beginning the treatment. Abdominal pain disaappeared. On treatment discontinuation, tumour size increased.
Studies of new drugs combined with VEGF-targeted agents are ongoing. Indiana University has developed a protocol combining oxaliplatin and bevacizumab in patients with refractory or relapsed germ cell tumours. Two ongoing phase II trials are currently investigating the effect of sunitinib in patients with refractory or relapsed germ cell tumours. It is difficult to evaluate new targeted drugs in this disease because chemotherapy is still the standard treatment. Trials comparing standard chemotherapy with or without targeted drugs are needed. Nevertheless, the number of patients concerned is too small to initiate phase III trials. On the other hand, even if a large amount of biological information is becoming available,38 there is insufficient biological background to identify specific targets. In our opinion it would be interesting to select specific clinical settings (e.g. growing teratoma for the study of differentiation mechanisms, refractory undifferentiated tumours for drug resistance and stem cell biology) and try targeted drugs while studying variations of molecular characteristics before and after therapy. To date, germ cell tumours have not really been the object of such translational studies.
Conclusion
Over the past 20 years, the BEP regimen has remained the standard of care for patients with germ cell tumours, with either three cycles of BEP or four cycles of EP in good-prognosis patients, or four cycles of BEP in those with intermediate or poor prognosis. No randomised trial has shown a superiority of other associations or HDCT over BEP for first-line treatment. More than 80% of patients are cured with a combination of chemotherapy and the resection of residual masses. Nevertheless, the prognosis of poor-risk patients is poor and trials with combinations of new drugs such as paclitaxel and oxaliplatin with BEP in first-line treatment are ongoing. In relapse, the situation differs because only 25% of patients are cured with the VeIP regimen. The results of first-line salvage treatments with the TIP regimen in patients with good risk of relapse are encouraging. New drugs are being developed for salvage treatment in cisplatin-sensitive, refractory or absolute refractory disease. The most active chemotherapy drugs as single agents are gemcitabine, oxaliplatin and paclitaxel. Targeted drugs are being evaluated in relapsed or refractory germ cell tumours.