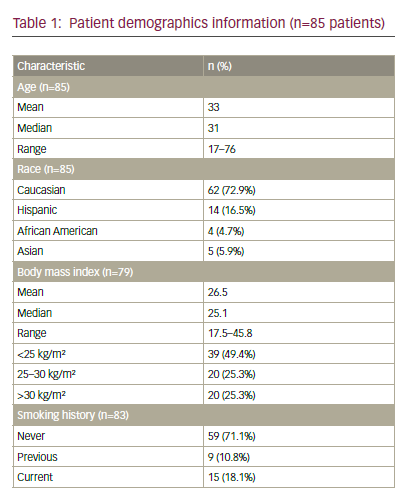
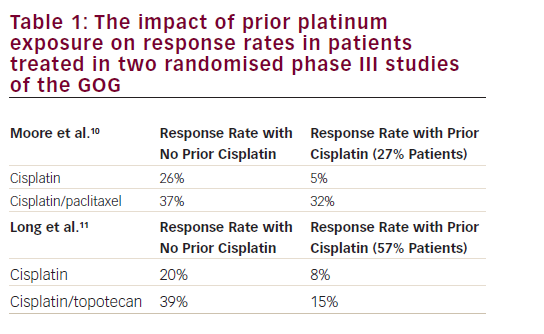
Endometrial cancer (EC) is the most common gynaecological cancer in the developed world, and in 2002 it was estimated that worldwide around 200,000 women were diagnosed with the disease.1 In Sweden, between 1970 and 2006 the age-standardised incidence increased by 37%, but because of an ageing population the number of cases increased by 80% during the same period.2 EC has a good prognosis, but per stage it is about the same as for ovarian cancer.3 In International Organization for Gynaecology and Obstetrics (FIGO) stage I there are subgroups with a high risk of micrometastatic disease. For example, patients with stage IC grade 3 disease have 79% five-year overall survival (OS) despite liberal use of adjuvant radiotherapy (RT).3 Four large trials have randomised patients to adjuvant pelvic RT or observation after surgery.4–7 All four failed to show an improvement in OS, despite the fact that RT prevented up to 80% of progressions in the irradiated field. Thus, most patients harbouring micrometastatic disease also have dissemination outside the irradiated field and there is a need for systemic adjuvant therapy, either added to or instead of RT.
To read full article please click here