Endometrial cancer is the most common gynaecological malignancy. The five-year survival rate is excellent: 84.3 % in Spain for all stages with complete treatment.1 The International Federation of Gynecology and Obstetrics (FIGO) stage is the independent criterion that best indicates the prognosis of this tumour. In the last FIGO Annual Report the five-year survival rate for surgical stage I was 90 %.2
In this article, we examine whether preserving fertility is a safe therapeutic option in selected cases of young women with endometrial cancer. We evaluate the characteristics of these young patients, selection of criteria and related problems, diverse treatment regimens, follow-up and reproductive outcome, using the evidence present in the literature. We consider young patients as those <45 years of age. Usually, diagnosis of endometrial cancer occurs in post-menopausal women after their sixth decade, with an average age of 61 to 64 years of age, depending on the series. The hormonal status of patients shows that only 11 % are pre-menopausal when diagnosed.1
Although it is uncommon in young patients, this malignancy is diagnosed in about 2–14 % of women ≥45 years of age. This broad age range is the reason for the variance in cut-off points for young patients in the different series. We diagnose this malignancy in women who have not borne children, and due to the challenge of childbirth, conservative treatment has been discussed in recent years as a safe therapeutic option for these patients. The current evidence is insufficient, but increasingly becoming more available via recent prospective studies and meta-analyses.
Characteristics of Young Patients
For a better assessment of selection criteria for conservative treatment, we consider different population characteristics. This is an initial point in recognising the behaviour of endometrial cancer in young women. The median age at diagnosis in this group of young women is 40 (range 31–45 years of age).
3</ Abnormal uterine bleeding is the onset symptom in most cases,3,4 causing delay and making diagnosis a more difficult task, which sometimes accounts for the confusion with dysfunctional uterine bleeding.
We reported an increased percentage of sterility and nulliparity (61 % of patients <45 years of age were nulliparous versus 24 % in the older group).5 These results are even lower than those reported by other physicians, such as Navarria et al., who found a 79 % incidence of nulliparous patients.6 Other authors have accounted for an increased incidence of endometrial cancer in patients with other hormonal disorders related to an excessive oestrogen exposure, such as anovulation, polycystic ovary syndrome and obesity, all considered risk factors for endometrial cancer in young women.4,7,8
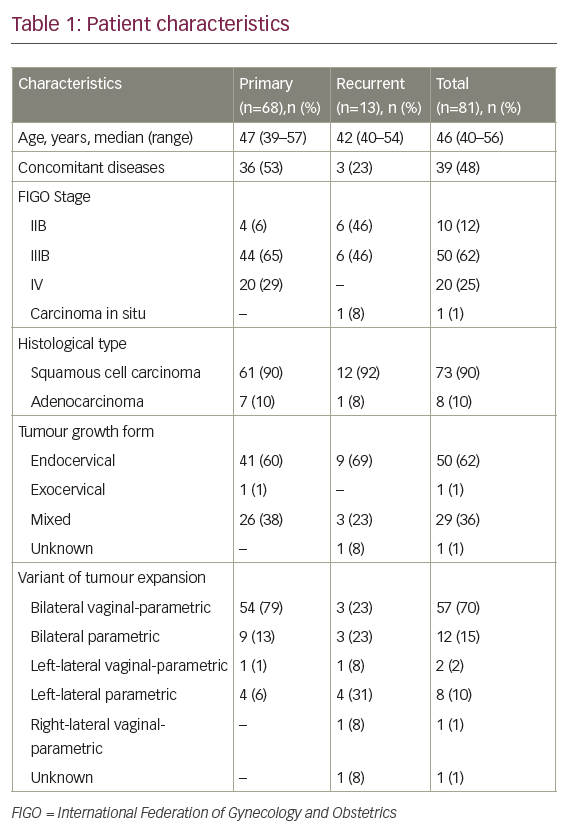
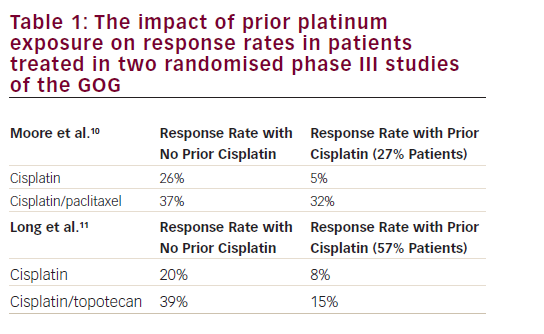
Some physicians emphasise the importance of a family history of malignancies, with 10–13 % of first-degree relatives affected with breast, colon, gastric, ovarian or endometrial cancer, or relatives affected with hereditary non-polyposis colorectal cancer (HNPCC).3,8 Previously, we described a higher rate of associated atypical hyperplasia in the young group (12.5 versus 2.4 %) and the histological type was more frequently grade 1 (62.5 versus 43 %) (see Table 1).1,5 The distribution of stages is nearly the same in both age groups, with a similar prevalence of stage I disease in both, although, in stage I, younger patients are more likely to present with disease confined to the uterus (Stage IA).5,9 Another important matter is ovarian disease, either as metastases from endometrial cancer or as synchronous malignancy. The incidence rate described for ovarian involvement in young patients with endometrial cancer is higher than in older women. We review in detail the literature below; nevertheless, one of the selection criteria is low-grade stage I disease, where ovarian metastases are rare. The incidence rate described for ovarian malignancies in all patients with stage I endometrial cancer is 5 %. The incidence of lymph node involvement is approximately 32 % in patients with ovarian metastases. In spite of this association, they are independent prognostic factors in clinical stage I endometrial cancer. The five-year disease-free survival rates reported for this patients group is 72.2 %.10
There are varying reports of a higher incidence of ovarian involvement, in a range from 5 to 29 %, in young women candidates for conservative treatment. Gitsch et al.3 found synchronous ovarian malignancies in 29.4 %, significantly higher than in patients older than 45. Crissman et al.4 reported co-existing tumours in 18.8 %; of these, 67 % were endometrioid type. However, Evans-Metcalf et al.
described a significant difference in synchronous ovarian malignancies between the two age groups in a univariate analysis, but multivariate logistic regression analysis revealed that age was not an independent risk factor, as previously believed; the real risk factor for synchronous ovarian and endometrial disease was nulliparity.9
Navarria et al. found a higher risk of synchronous ovarian malignancies in the young group compared with patients >65 years of age (14 versus 2 %); most of them were found during surgery.6 Soliman et al. found a higher rate of synchronous ovarian cancer (19 %), 78% of these having an abnormal appearance at the time of surgery.8 Other authors reported a similar incidence of benign manifestations: between 9 and 15 % of cases at the time of surgery.11 Walsh et al. reported co-existing ovarian malignancies in 25 % of cases. In accordance with other authors, in their report the principal histological type was endometrioid. It is surprising that in 88 % of cases described by these authors, the pathological findings suggested synchronous endometrial and ovarian primary tumours, and the remaining cases were metastases (see Table 2).11
Taking into account this increased risk, conservation of the ovaries is important to avoid hormonal deprivation and premature menopause with long-term morbidity in the youngest patients. Physicians must consider the risks and benefits when evaluating ovarian preservation.
Selection Criteria
The most important criterion for an optimal clinical outcome is a correct selection of candidates for conservative therapy. The first and indispensable condition for sparing fertility must be a precise diagnosis of a well-differentiated endometrial carcinoma, since only grade 1 tumours can be selected for conservative treatment. In addition, in these well-differentiated tumours, the progesterone receptors necessary for response to medical treatment exist in higher concentration than in poorly differentiated tumours (85–90 % versus 55–60 %). This pathological feature gives these patients a better survival rate than other groups. There must be no cervical or myometrial invasion; therefore, only superficial tumours can be selected for conservative management.
Myometrial invasion and histological grade are important prognostic factors of recurrence, as described by Morrow et al.
12
As the grade becomes higher and myometrial invasion appears, the rate of lymph node involvement rises from 2 to 34 % for pelvic node involvement and from zero to 25 % for para-aortic nodes. When pelvic and para-aortic nodes are involved, recurrence rates are described as 25 and 60 %, respectively. The lymph node metastasis rate described in the literature is 3–5 % for patients with non-invasive grade 1 endometrioid disease.10 Histological type is also an important prognostic factor, so we exclude all high-risk variants of endometrial cancer, such as clear cell and papillar serous tumours.
In the past few years there has been increasing research interest in other features of endometrial cancer capable of predicting clinical outcome and response to hormonal therapy. These tumour characteristics are based on immunohistochemical analysis of molecules such as phosphatase and tensin homologue (PTEN), phospho-Akt, p53, and oestrogen and progesterone receptors. PTEN is described as a tumour-suppressor gene, mutated in 30–55 % of endometrial carcinomas.
By upregulating PTEN proteins, positive discussion surrounds the possibility of an anti-tumour effect of progestins. This mechanism is associated with a downregulation of Akt phosphorylation. Minaguchi et al. found that the presence of PTEN-null tumour cells and weak phospho-Akt is associated with a failure of progesterone treatment and the recurrence of the tumour using the conservative approach (see Figure 1).13 Another important molecular feature described in recent years has been DNA mismatch repair (MMR) protein defects. Garg et al. detected that MMR loss is associated with high-grade tumours and unfavourable clinical results.14 These tumours also show lower oestrogen/progesterone receptor expression and more aggressive behaviour. These authors described how 22 % of patients with abnormal MMR died of disease compared with 4 % in a group without loss of DNA MMR proteins.14 The study of MMR can also be performed on young patients candidate for fertility preservation, and if they show loss of MMR we disqualify them for conservative treatment.
Problems in Patient Selection
Diagnostic tests are essential in patient selection, but difficulties do arise and we are exposing important points of the diagnosis process. Biopsy with Cornier pipelle, office biopsy or curettage could be insufficient, with the consequent false-negative rate; hysteroscopy is more adequate in these cases. Hysteroscopy permits the evaluation of the endometrial cavity, and biopsy of suspicious lesions. The accuracy of hysteroscopy is sufficient to diagnose endometrial cancer or hyperplasia in the case of abnormal bleeding, with a sensitivity rate of 86.4 % and specificity rate of 99.2 %, although the accuracy seems to be higher in diagnosing cancer than in excluding it.15 There are controversies with physicians who defend dilatation and curettage as opposed to hysteroscopy because of the risk of spreading endometrial cancer cells into the abdominal cavity, giving rise to stage IIIA cancer with the subsequent adjuvant treatment; the clinical impact remains unclear.16
There are severe difficulties in obtaining a consistent diagnosis to differentiate between atypical hyperplasia and well-differentiated carcinoma. In the cases reviewed by Crissman et al. 41 % of the cases with endometrial were overdiagnosed.4 The current classification by Kurman and Norris has been discussed in different series comparing its reproducibility. A review concluded by Zaino et al. in a Gynecologic Oncology Group (GOG) study described the reproducibility of the diagnosis of endometrial hyperplasia. They reported a complete diagnostic agreement in only 40 % of cases, with the greatest concordance in the diagnosis of normal tissue and endometrial cancer. The main problem here was that the diagnosis of atypical hyperplasia was only fair, with a low correlation coefficient (k=0.28) and reproducibility. However, the kappa coefficient for endometrial cancer diagnosis was 0.51, with moderate reproducibility. Therefore, there is an intrinsic problem in the reproducibility of atypical hyperplasia diagnosis, influenced by classification criteria or small sample size.17 From our experience, a pathologist with expertise in gynaecological pathology must perform the histological study. Moreover, the slides should be studied by at least two gynaecological pathologists. The clinical absence of myometrial invasion is another important selection criterion. However, myometrial invasion is difficult to determine by curettage, hysteroscopy or biopsy. We find in our daily clinical practice that the best assessment of myometrial invasion is by contrast-enhanced magnetic resonance imaging (MRI); this is in accordance with the meta-analyses of radiological evaluation of myometrial invasion carried out by Kinkel et al.18 In this report, contrast-enhanced MRI permits the more accurate evaluation of myometrial invasion, and cervical and nodal involvement.
The disease must be confined to the uterus and, because of the frequency of synchronous ovarian tumours, an accurate evaluation is important. Ovarian tumours have an abnormal appearance at the time of surgery in 78 % of cases.8 Therefore, laparoscopy must include an examination of peritoneal cytology and a careful evaluation of the ovaries, peritoneal cavity, omentum and pathological lymph nodes. Some authors recommended lymph node sampling at the time of laparoscopic evaluation.19
The accuracy of MRI can probably be complemented by other imaging techniques such as positron emission tomography/computed tomography (PET/CT). Used in other gynaecological malignancies, this is very helpful in the staging and diagnosis of ovarian disease. Horowitz et al. described a high specificity of 98 %. Therefore, this diagnostic technique has potential to assist in the pre-operative management of this malignancy.20
In summary, for proper selection of patients for conservative treatment we must perform an accurate diagnosis for early disease stage, including well-differentiated carcinoma, no myometrial invasion demonstrated by contrast MRI and absence of suspicious pelvic or para-aortic nodes nodes. Absence of synchronous ovarian tumours is another important requirement. We should consider performing a diagnostic laparoscopy before including the patient in the protocol. Determination of serum ovarian tumour markers should be included in the pre-operative test.21
Treatment
The conservative approach to endometrial cancer is based on systemic hormonal treatment. As an exception, some authors have proposed local surgical excision by hysteroscopy or repeated curettage, as well as a progesterone intrauterine device (IUD) as local treatment.
The first options used by most authors are progestin derivatives; about 75 % of conservative treatment includes progestin therapy with medroxyprogesterone (MPA) or megestrol. Other hormonal therapies include luteinising hormone-releasing hormone (LHRH) agonists, anti-oestrogens and aromatase inhibitors. The articles available included studies with different hormonal drugs, dosages and durations of treatment. Next, we summarise the most common regimen described in the literature in order to facilitate its clinical application. Progesterone derivatives have been used in endometrial cancer for years because it is a hormone-dependent tumour. Progesterone can stimulate histological differentiation of the glandular epithelium into secretory structures, inhibit the oestrogenic effect and suppress cell proliferation.22 Progesterone therapy can be used in different protocols. MPA is the most common regimen, used in 50–57 % of cases in daily dosages ranging from 200 to 800 mg. Another important regimen is megestrol, used in 23–24 % of cases, in dosages from 10 to 400 mg.8,23,24 The levonorgestrel intrauterine system has been used as local treatment but there have been some recurrences.24 Most reports are in elderly patients with comorbidities, used alone or in young women, in combination with oral progestins. Until recently, evidence for the use of an IUD for conservative treatment in young patients was scarce. However, there is a recent study from Minig et al. who tested the efficacy of levonorgestrel IUD plus gonadotropinreleasing hormone (GnRH) for conservative treatment in young women with atypical endometrial hyperplasia (AEH) or FIGO stage IA endometrioid endometrial cancer. The complete response rate was 95 % in patients with AEH and 57.1 % in those with endometrial cancer. In the case of cancer, the recurrence rate was 14.3 % and the progression rate 28.6 %; these results are in accordance with rates in the literature.25
Other Medical Treatments
There are reports using growth hormone-releasing hormone (GHRH) agonists, although these were used in second-line hormonal therapy after progestins failed. Aromatase inhibitors are recommended in obese patients where it is necessary to avoid peripheral oestrogen production. Tamoxifen used in combination with progestins provides improved downregulation of hormonal receptors compared with progesterone alone.
Surgical Treatments
Most reports described combined therapy, comprising hysteroscopic resection followed by oral progestins. Experience is scarce; there are successful cases and also recurrences. Actually, conservative hysteroscopic resection as the sole therapy is not recommended.24 In the worldwide literature on this conservative approach, the most relevant review articles were published by Ramirez et al.26 and Chiva et al.,5,23 and the most recent by Erkanli et al.24 The clinical outcomes of these reviews are in agreement. The overall response rate was 74–76 %. The median time to response was 12 weeks with a median duration of treatment of 24 weeks. In the group of patients with relapse (24–25 %), the average relapse time was 19–20 months. The average duration of hormonal therapy was six months; most authors consolidate treatment, in spite of a negative biopsy after the first 12 weeks of hormonal therapy. Six months seems to be a logical time to cease conservative treatment when progression or persistent disease is present, based on the latest review where a mean time for confirmation of persistent/progressive disease was calculated as 6.7 months.24
Some authors have reported repeat treatment after relapse. Ramirez et al. described how 47 % of patients with recurrence were re-treated with progesterone; of these, 72 % experienced complete response. These results are very similar to those of Chiva et al. and Erkanli et al., where the re-treatment rate was 30 % for both studies and the complete response rate was 80 %. Nevertheless, in most of the articles reviewed, the patients who relapsed or did not respond underwent full surgical treatment.
In summary, one-half of patients can preserve fertility due to a complete response to hormonal therapy. Twenty-five per cent will experience a temporary response, and the remaining one-quarter will have no response to conservative therapy (see Figure 2).23 Unfortunately, there have been some cases of death reported in patients with metastatic disease. In all of these cases, there was an initial complete response. It is possible that this incidence could be greater, because unfavourable cases are not always published. We cannot prove if these patients would have died even if they had received the standard therapy. But what is very important is that we should make extremely clear to patients the risks that they run in preserving their fertility (see Table 3).Reproductive Outcome
The pregnancy rate described in the literature by different authors is similar, between 35 and 40 %.23,24 Conservative treatment of endometrial cancer is not always followed by pregnancy, because in many cases the cause of the endometrial malignancy, such as a hormonal disorder, is linked to infertility. Around 60 % of these young patients were nulliparous when diagnosed. In our previous review, 66 % of pregnancies were obtained by assisted reproductive technologies.5 This rate is much higher than the percentage reported in the latest review,24 where 17.9 % of patients needed these techniques.
Follow-up
In spite of recent important reviews, the experience available is insufficient to establish a consistent follow-up protocol, because they are based on small series and reported cases.
Based on the median response time of 12 weeks, endometrial biopsy is recommended every three months, until a complete response or pregnancy. In our experience, we recommend performing this biopsy by hysteroscopy with a complete evaluation of the uterine cavity. In case disease persists, we can continue with treatment for another 12 weeks. A response should be obtained after six months of treatment; if not, standard surgery must be performed.
A negative result permits patients to become pregnant, with close surveillance until childbirth. The frequency with which biopsy is performed prior to pregnancy is difficult to determine; it is advisable to perform intermittent biopsies in the pre-ovulatory part of the cycle every three or four months. An appropriate follow-up protocol, including biopsy frequency, imaging techniques and use of hysteroscopy, needs to be developed for early detection of local and distant recurrences.
Conclusion
Conservative treatment of young patients with endometrial cancer is a valid therapeutic option if childbearing is not completed. For an adequate oncological outcome, we must apply strict selection criteria. Study of a biopsy by two gynaecological pathologists may help to obtain an accurate diagnosis. Patients need special surveillance of the ovaries due to the risk of concomitant malignancy.
Patients must know all the risks and benefits involved in this conservative approach, as well as the use of standard surgical treatment when reproductive desires are satisfied.
As it is an uncommon situation, the evidence based on retrospective analyses is scarce. For this reason multicentre prospective studies must be performed.