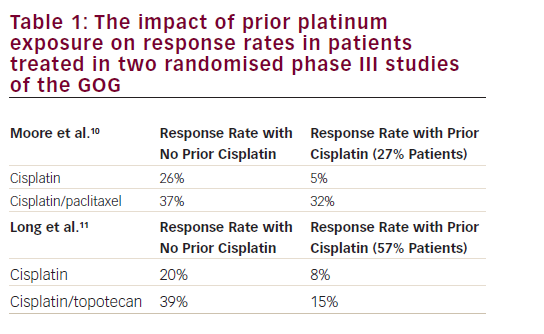
Despite many years of trials, there is no single evidence-driven best regimen for newly diagnosed, advanced (defined as stage II or greater, i.e. extra-ovarian spread) epithelial ovarian cancer and its counterparts, fallopian tube cancer and primary peritoneal cancer. Instead, there is agreement that the essential components of treatment are surgical debulking and chemotherapy containing a platin analog, ideally carboplatin, with the use of a taxane at some point. The route of chemotherapy (intravenous or intraperitoneal), timing of the taxanes (concurrent or sequential/delayed), frequency, dose, number of cycles, timing of surgery (upfront or delayed, i.e. interval debulking), and degree of surgical aggressiveness are left to the treating physician’s interpretation of the data. The regimens that the provincial Gynecology Tumor Group of the British Columbia Cancer Agency have decided are optimal are listed in Table 1 (two ‘standard’ regimens with two alternatives if there is undue toxicity on treatment or a desire to avoid a particular toxicity; elderly—those over 80 years of age—and frail patients will usually start with singleagent carboplatin). The evidence that led to these funded options being chosen is also shown in Table 1.
Surgery Is Therapeutic
Intuitively, it makes sense that debulking surgery would be therapeutic as it removes cancer and, importantly, potentially resistant clones. Griffiths demonstrated in 1975 that the size of the cancer remaining after surgery was prognostic in ovarian cancer.1 This retrospective review could not differentiate between a true therapeutic effect of surgery and just an effect of tumor biology (resectable cancer is less aggressive). To differentiate between these effects, one would need to compare chemotherapy alone versus surgery plus chemotherapy. Such a study has not been performed; instead, there are two large phase III studies that have looked indirectly at the therapeutic role of surgery. They compared outcomes in women who, after an initial attempt at debulking followed by chemotherapy, were randomized to either a second debulking and more chemotherapy or to chemotherapy alone.2,3 The European study showed improved survival in those with the second debulking (median overall survival increased from 20 to 26 months), whereas the US study did not. Are there any possible explanations for the discordance? In the US study significantly more patients had smaller-volume disease after primary surgery and the chemotherapy was superior, so would stand to potentially benefit less, especially if chemotherapy had already ‘debulked’ them, and more salvage therapies were available to improve overall survival. Finally, the biology of the disease left after initial surgery in the two studies may have been different. The US policy was to attempt maximal debulking, which was not necessarily the case in the European study. Thus, cancer left behind in the US study was likely more ‘virulent’ and less susceptible to treatment.
The question of the optimal timing of surgery has been resolved. Delaying the surgery until after the third or fourth cycle of chemotherapy had no negative impact on survival, but was less morbid, quicker, and easier for the patient.4
Who should perform the surgery? The survival data show that expertise matters, i.e. women survive longer if managed by gynaecological oncologists or gynecologists with training in cancer surgery.5,6
How aggressive should the surgeon be in removing cancer? The underlying concept is that the less visible cancer is left, the better the outcome. There is an increasing body of opinion, without definitive data, that maximal surgical effort should be carried out including such things as diaphragmatic stripping, splenectomies, etc. An intriguing post hoc report from a European study showed that ‘aggressive’ surgery benefits only those with lesser amounts of disease.7 Presumably, tumor biology has more impact on outcome than treatment in the more advanced/ aggressive scenario.
Intravenous Carboplatin plus a Taxane Is the Optimal Current Regimen
Gynecologic Oncology Group (GOG) 111, subsequently confirmed by OV 10, revolutionized the chemotherapy of ovarian cancer. The combination of cisplatin–paclitaxel proved superior to cisplatin– cyclophosphamide, the previous North American gold standard, with a consistent absolute survival improvement at all time-points of about 11% over six years of follow-up, which translates into 27 versus 16% survival at six years.8 Paclitaxel was given as 135mg/m2 over 24 hours in GOG 111 and as 175mg/m2 over three hours in OV 10. These are equivalently efficacious doses/schedules when used in the relapse setting.9 As such, the more convenient three-hour schedule has been universally adopted.
Cisplatin is a cumbersome and toxic drug to deliver. Carboplatin is much more user-friendly and in combination with paclitaxel has equivalent efficacy, more manageable toxicity, and better quality of life compared with cisplatin.10–12 There are no data that prove the optimal dose of carboplatin. The Gynecologic Cancer Intergroup’s choice of dose in their multinational trials represents the consensus opinion. The most frequent dosing of carboplatin was an area under the curve (AUC) value of 5—dose in mg = (glomerular filtration rate [GFR] + 25) x 5—based on the GFR calculated from the serum creatinine, plus paclitaxel 175mg/m2 over three hours repeated every three weeks for six cycles with the option of continuing for those in partial remission.
There are limited modern data on the optimal number or frequency of cycles. Eight cycles of single-agent platin were not superior to five cycles, and this is concordant with older results.13 A retrospective singleinstitutio study found no difference in outcomes with every-three weeks versus every-four-weeks treatment.14 This parallels our experience and as such our protocol allows for treatment every three or four weeks. Increasing the dose of either carboplatin or paclitaxel or giving the paclitaxel by 96-hour infusion did not improve outcomes.15–17 Docetaxel at a dose of 75mg/m2 can replace paclitaxel with no loss of efficacy but a differing toxicity profile, e.g. more neutropenia and edema but less myalgia, alopecia, and neuropathy, which lets us tailor our treatment to the individual patient to a certain extent.18 Sequential Use of a Platin Followed by a Taxane
No phase III trials have addressed this question specifically; however, GOG 132 did so inadvertently.19,20 In this study, women were randomized to cisplatin alone, paclitaxel alone, or the combination of cisplatin plus paclitaxel. The progression-free survival was 16, 11, and 14 months, respectively. Median overall survival was 30, 26, and 27 months, respectively, i.e. cisplatin alone was as good as cisplatin–paclitaxel. However, 40% of patients on the cisplatin alone arm crossed over to paclitaxel (52% of them) or another treatment before progression, i.e. a de facto sequential approach; similarly, 44% had early switching to a platin or other treatment from the paclitaxel arm. One interpretation of these results is that a sequential approach is as good as initial combination treatment. The downside to this is that it requires more treatment cycles, with related costs to the patient and the system, to achieve the same result. Hence, combination remains the gold standard, except where its increased toxicity is a relative contraindication, for example in the elderly. Our single-agent choice is carboplatin, as it is less toxic than cisplatin and GOG 132 demonstrated that single-agent paclitaxel was inferior to cisplatin in terms of response rate and time to first progression.
Is Single-agent Carboplatin as Good as Carboplatin–Paclitaxel?
An alternate explanation of GOG 132 is that paclitaxel has little additional therapeutic activity when cisplatin is used and that the early cross-over after cisplatin had no effect. Support for this possibility was that the progression-free survival for cisplatin and cisplatin–paclitaxel was the same despite 60% of the cisplatin monotherapy patients not crossing over early to another treatment. If paclitaxel had added much to the treatment, one would have expected a longer initial time to progression with the combination and then ‘catch-up’ within the singleagent arm so that the overall survival was the same.
This interpretation is supported by the large randomized ICON 3 study, which compared carboplatin versus carboplatin–paclitaxel (the cyclophosphamide–doxorubicin–cisplatin arm having been dropped during the study subsequent to the ICON 2 results).21,22 The hazard ratio in favor of carboplatin–paclitaxel was 0.98 for overall survival (an absolute benefit of only 0.7 months) and 0.93 for overall survival (an absolute benefit of one to two months for the median; p=0.19)— neither statistically significant nor clinically relevant. Not surprisingly, these results were unpopular with the ‘combination’ supporters, ourselves included. A multitude of possible explanations, essentially methodological, other than a true result have been cited as the reasons for this.23,24
The issue of single-agent carboplatin versus combination is still unresolved, although the practical reality is that both in North America and in clinical trials, including the ICON collaborators, carboplatin–paclitaxel is the standard therapy. The recent experience with triple-drug combinations (carboplatin–paclitaxel plus any of topotecan, liposomal doxorubicin, epirubicin, or gemcitabine), none of which were superior to carboplatin–paclitaxel, is relevant to this question. The most likely reason for the negative results was that these third drugs had minimal ability to kill truly platin-refractory cells.25 Paclitaxel is not obviously superior to any of these other drugs and so the likelihood is that it also has limited ability to kill truly refractory cells, hence the lack of additional effect in ICON 3.
Despite all of this, in British Columbia we continue to recommend the combination of carboplatin–paclitaxel as our standard first-line treatment for a number of reasons. First, in platin-sensitive first relapse, combinations are superior to single-agent carboplatin.26,27 This is a more ‘selected’ group of patients than the newly diagnosed mixture of both the curable and incurable who have differing degrees of chemotherapy sensitivity, and so small benefits may be more readily apparent. Second, post hoc subset analysis of 1CON 3 showed a trend toward superior outcome with the combination in the cohort with residual tumor >2cm, of a similar degree to the trends in GOG 111 and OV 10. Finally, a recent abstract from the American Society of Clinical Oncology (ASCO) has demonstrated improved outcomes by changing the scheduling of paclitaxel, implying an effect of paclitaxel.28 In this study, the control arm was carboplatin (AUC 6) plus paclitaxel 180mg/m2 over three hours every three weeks for six cycles. The experimental arm replaced the q 3 week paclitaxel with weekly paclitaxel 80mg/m2 on days one, eight, and 15. This maneuver significantly increased both median progressionfree survival (28 versus 17 months) and two-year overall survival (84 versus 78%). We are currently discussing whether this should become our new standard.
Intraperitoneal Chemotherapy
This is another area where there is no universal consensus despite the weight of evidence.29,30 The major problem is that the trials carried out in the taxane era changed not only the route but also the dosing and frequency. As such, one cannot say which component was the important one. Be that as it may, the results are still superior, so not only in British Columbia but also across Canada intraperitoneal-based therapy has been adopted. The basic premise behind intraperitoneal therapy is that much higher peak doses (20-fold higher for cisplatin and 675-fold higher for paclitaxel) that persist for longer (AUC 10-fold greater for cisplatin and 1,000-fold greater for paclitaxel) can be achieved in the peritoneum, the predominant site of ovarian cancer, compared with the plasma. Due to subsequent re-circulation through the venous system, plasma levels are similar to those achieved with standard intravenous dosing. Overall drug levels within the cancer are higher and more uniformly distributed when the intraperitoneal route is used.
The randomized trials were conducted in women with stage III disease (intra-abdominal spread) with residual tumor <1cm in the maximal size left after initial debulking. The absolute benefit in median overall survival with the intraperitoneal protocols was about 12 months (60 versus 48 months) in a meta-analysis of the eight trials.29
Toxicity/delivery problems due to both the chemotherapy (predominantly cisplatin-related) and the type of catheter utilized was excessive. In the Armstrong study (GOG 172), the average number of cycles delivered was three, not the planned six. Only 42% of patients received all six cycles. Despite this, the absolute benefit in median overall survival was still 16 months (66 versus 50 months). Our approach has been to use this particular protocol but to substitute carboplatin for cisplatin (see Table 1) and use 9.6Fr single-lumen venous access catheters inserted over the lower ribs in the anterior axillary line and tunneled subcutaneously to the lower abdomen. As a result, 80% of our patients have received all six cycles. The substitution of carboplatin for cisplatin is being formally tested in Japanese and Canadian/European phase III studies. Given the switch away from up-front surgery to delayed surgery that will arise from the OV 13 results, we also use the same protocol but with just three cycles once delayed optimal debulking (<1cm) has been carried out. There are no data to support or refute this approach, and the abovementioned Canadian/European study is a test of this. Maintenance Paclitaxel
The use of maintenance paclitaxel after successfully completing firstline cisplatin or carboplatin–paclitaxel has produced mixed results. In the original study comparing 12 versus three cycles of maintenance using paclitaxel 135mg/m2 every four weeks in women after achieving a complete response (normal markers, normal imaging), the median progression-free survival improved to 28 months from 21 months. The study was stopped early due to pre-specified stopping rules for superiority. No overall survival benefit was reported, but the sample size was small. However, a subset analysis revealed a significant survival benefit for those whose cancer antigen 125 (CA-125) on study entry was <10 units/ml.31 An ongoing GOG study is investigating this formally. Toxicity—predominantly neuropathy and continuing alopecia—were more common with the 12-cycle arm. A second study has also been carried out.32 Six cycles of maintenance paclitaxel (175mg/m2 q3w) was compared with no further therapy. Again, there was an improvement in median progression-free survival (34 versus 30 months), but no significant difference in overall survival at three years (78 versus 88%), achieved at the cost of significant sensory neuropathy and the additional time involvement. Our Tumor Group regards this additional ‘cost’ to the patient for a potential improvement in progression-free survival but with no overall survival benefit as being of insufficient value to recommend maintenance therapy with paclitaxel.
Failed Approaches (Phase III Results)
Adding a third drug—one of gemcitabine, topotecan, epirubicin, or liposomal doxorubicin as triplets, sequential doublets, planned sequential, or consolidation—has not led to any survival benefit, with lack of significant non-cross-resistant activity with platins being the most plausible reason.25 High-dose chemotherapy using autologous bone marrow transplant or peripheral stem cells has been tested. Conceptually, this is similar to using intraperitoneal therapy, in that higher doses of chemotherapy can be given. However, unlike the intraperitoneal route, the dose increase achievable is modest due to dose-limiting non-hematological toxicities. Randomized studies have not demonstrated any survival benefits for either progression-free or overall survival.17,33
A variety of immune manipulations in addition to standard chemotherapy have been tested and have failed. Oregovomab is a murine antibody specific for CA-125 that induces tumor-specific humoral and cellular immunity. In the original study, patients who achieved a complete response with their initial platinum-based chemotherapies were randomized to either oregovomab or placebo maintenance.34 There was no improvement in time to relapse for the overall population, but a subset with <2cm residual tumor postoperatively plus faster response to chemotherapy did perform better. A subsequent randomized, blinded study was carried out in this subset, and there was no additional benefit in time to relapse.35 Interferon has also been tested. In the original small study, interferon-γ was given concurrently with cisplatin and cyclophosphamide, with an improvement in progression-free but not overall survival.36 The study was repeated but with a much larger sample size and using carboplatin plus paclitaxel. The study was stopped early because of an excess of deaths in the experimental interferon-containing arm.37 Interferon-α used as maintenance therapy also had no additional survival benefits.38
The role of irradiation has also been studied. Four hundred and forty-seven women with a negative second-look laparoscopy after initial chemotherapy were randomized either to observation or to a single intraperitoneal dose of yttrium-90-labeled human milk fat globulin 1 (HMFG1) murine antibody. There was no difference in time to relapse or overall survival.39 In contrast, a smaller Scandinavian study from the pre-taxane era showed a benefit for whole abdominal irradiation (four cycles of chemotherapy plus irradiation versus 10 cycles of chemotherapy).
In the subset who were in pathologically complete remission, the use of irradiation improved five-year progression-free survival to 86% from 56% and overall survival to 69% from 57%. Confoundingly, if there was microscopic residual tumor, these figures were reversed, i.e. chemotherapy alone was superior;40 this dichotomy raises the distinct possibility of a false-positive result. Whole abdominal irradiation has not been adopted anywhere as a component of standard therapy.
Finally, inhibiting multidrug resistance using a cyclosporine analogue allowed for lower dosing of paclitaxel but with no survival benefit.41 The use of a matrix metalloproteinase inhibitor—i.e. targeting enzymes that degrade extracellular matrix and allow metastases—as maintenance also had no benefit.42 ■